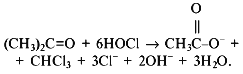Hypochlorite Reacts with an Organic Hydroperoxide Forming Free Radicals, But not Singlet Oxygen, and Thus Initiates Lipid Peroxidation
O. M. Panasenko,1,2 J. Arnhold,3 and J. Schiller3
1Institute of Physico-Chemical Medicine, ul. M. Pirogovskaya 1a, Moscow, 119828 Russia; E-mail: panasenko@glas.apc.org2To whom correspondence should be addressed.
3Institute of Medical Physics and Biophysics, Leipzig University, Liebigstr. 27, 04103 Leipzig, Germany; fax: (0341) 971-5709.
Submitted April 22, 1997; revision submitted June 11, 1997.
The mechanism of the reaction of hypochlorite with t-butyl hydroperoxide as a model organic hydroperoxide was studied. The reaction produces chemiluminescence with rate constant 13 ± 2 mM-1·sec-1. The chemiluminescence of this reaction was compared with that of the hypochlorite reaction with H2O2 where singlet oxygen (1O2) is formed. In the hypochlorite reaction with H2O2, the effect of hypochlorite concentration on the integrated chemiluminescence intensity is quadratic; a red filter with transmission >600 nm did not significantly decrease the chemiluminescence intensity; substitution of D2O for H2O increased the luminescence intensity 10-fold; infrared monomol emission was observed at 1270 nm. These results confirm the formation of 1O2 during the hypochlorite reaction with H2O2. However, when t-butyl hydroperoxide was used instead of H2O2, the concentration effect significantly differed from quadratic, and the red filter decreased the luminescence intensity by ~99%; D2O slightly decreased the luminescence intensity. Finally, addition of t-butyl hydroperoxide to hypochlorite was not associated with monomol emission of 1O2 in the infrared region. The data exclude the possibility of singlet oxygen formation in the hypochlorite reaction with the organic hydroperoxide. According to 1H-NMR spectroscopy, di-t-butyl peroxide is the main product of the hypochlorite reaction with t-butyl hydroperoxide; its production can be explained by radical formation, i.e., by generation of t-butyloxy radical. t-Butyl hydroperoxide and cumene hydroperoxide promoted hypochlorite-induced lipid peroxidation of phospholipid liposomes. The free radical scavenger butylated hydroxytoluene completely inhibited this effect. The data suggest that organic hydroperoxides, always present in certain amounts in vivo, may be the intermediates that interact with hypochlorite-forming free radicals which are initiators of lipid peroxidation.
KEY WORDS: hypochlorite, lipid peroxidation, singlet oxygen, free radicals, hydroperoxide.
Oxidation of human blood lipoproteins (LP) is an important factor in the development of atherosclerotic lesions of blood vessels [1, 2]. Interaction of native LP with active oxygen forms including hypochlorous acid (HOCl) and its ionized form hypochlorite (OCl-) is one of the major reasons for the appearance of oxidized LP in the blood; HOCl/OCl- is formed in the reaction of hydrogen peroxide with chloride catalyzed by myeloperoxidase which is secreted by stimulated phagocytes [3]. It was demonstrated that exogenous hypochlorite or hypochlorite produced in myeloperoxidase reaction is potent a oxidizer and can activate lipid peroxidation (LPO) thus modifying human blood LP [4-8]. The modification of low density lipoproteins (LDL) induces their atherogenic properties so that these LDL are more actively absorbed by macrophages which are subsequently transformed into foam cells; this is one of the most important characteristics of early stages of atherosclerosis [2, 9].
The mechanism of hypochlorite-induced LPO is unknown. It was demonstrated that other active oxygen forms--hydrogen peroxide (H2O2), superoxide anion-radical, and iron ions that could be present in the LDL incubation medium containing hypochlorite--did not participate in the initiation of HOCl/OCl- induced LPO [10, 11]. HOCl/OCl- reacts with unsaturated bonds of the acyl chains of phospholipids [12, 13] but this reaction is not associated with LPO activation. Recently, it was shown that hypochlorite reacts with organic hydroperoxides included into phosphatidylcholine liposomes [14]. The reaction mechanism is unknown. However, hypochlorite reacts with hydrogen peroxide according to the equation [15]:
In the present work we studied whether the hypochlorite reaction with an organic hydroperoxide can be the source of the LPO initiators and whether 1O2 is formed similar to reaction (1) or that the reaction mechanism is different.
MATERIALS AND METHODS
The following reagents were used: t-butyl hydroperoxide, cumene hydroperoxide, t-butanol, di-t-butyl peroxide, egg phosphatidylcholine, D2O, and CDCl3 from Fluka (Switzerland); H2O2 and butylated hydroxytoluene (BHT) from Merck (Germany); hexamethylene from Aldrich (USA); hypochlorite from Sigma (USA); and 2-thiobarbituric acid (TBA) from Serva (Germany). t-Butyl hypochlorite was synthesized by bubbling gaseous chlorine through an alkaline solution of t-butanol [17]. All solvents for UV spectroscopy were from Fluka (Switzerland).
The concentration of hypochlorite and H2O2 were assayed spectrophotometrically at 290 (epsilon = 350 M-1·cm-1, pH 12) and 230 nm (epsilon = 74 M-1·cm-1), respectively [18, 19].
The kinetics of the hypochlorite reaction with t-butyl hydroperoxide was measured spectrophotometrically by monitoring the decrease in optical density at 290 nm (absorption maximum of OCl- [18]). The reaction rate constant was calculated using the initial segments of the exponential kinetic plots (first 0.1 min with automatic registration of optical density every 0.01 min; pseudo-first order reaction); t-butyl hydroperoxide was present in 5-12-fold molar excess over hypochlorite.
Chemiluminescence was measured by an AutoLumat LB 953 apparatus (Berthold, Germany). Hypochlorite solution (200 µl, final concentration 0-50 mM) in 100 mM carbonate buffer (pH 9.5) was added into the thermostatted (37°C) chemiluminometer cuvette. H2O2 (50 µl, final concentration 0.2 M) or t-butyl hydroperoxide (final concentration 40 mM) were injected and integral chemiluminescence intensity was registered for 10 sec. Some experiments were performed under similar condition using D2O as the solvent. In other experiments, a red filter transmitting only light of wavelength greater than 600 nm was placed between the cuvette and the detector.
Monomol emission of 1O2 was registered in the infrared region (1270 nm) at 37°C using a detector based on a germanium photodiode cooled to the temperature of liquid nitrogen [20].
The hypochlorite reaction with t-butyl hydroperoxide was performed at room temperature in 10 mM phosphate buffer (pH 7.4) prepared using deuterated water (D2O). Hypochlorite (10 mM) was mixed with 10 mM t-butyl hydroperoxide and incubated for 15 min. Deuterated chloroform CDCl3 (1 ml) was added to 1 ml of the reaction mixture and thoroughly mixed; the mixture was separated by centrifugation at 1000g for 3 min. The contents of the reaction products in D2O and CDCl3 phases were assayed by 1H-NMR. 1H-NMR spectra were recorded using an AMX-NMR spectrometer (Bruker, Germany) at 300.13 MHz with 64-scan signal accumulation. Chemical shifts of the signals were determined using the sodium salt of 3-(trimethylsilyl)-1-propane sulfonic acid (in D2O) of chloroform (in CDCl3). Signals were identified by comparison with chemical shifts of 1H-NMR spectra of individual compounds and by increase in the signals in the reaction product mixture after addition of the compounds.
Multilayer liposomes were prepared from egg phosphatidylcholine in 10 mM phosphate buffer (pH 7.4) containing 140 mM NaCl by dispersion of the dry phospholipid film for 30 sec; the film was formed by evaporating the phospholipid solution in chloroform under vacuum. Liposomes were incubated with hypochlorite for 40 min at 37°C. The liposome concentration in the incubation medium was 2 mg/ml and hypochlorite was 0-1.5 mM. In some experiments, liposomes were formed and incubated with hypochlorite in the medium prepared using D2O. The concentration of TBA-reactive products of LPO was assayed spectrophotometrically [4] and expressed as nmoles malonic dialdehyde (MDA) per mg phospholipid (the molar extinction coefficient of the MDA--TBA complex at 352 nm is 1.56·105 M-1·cm-1 [21]).
RESULTS
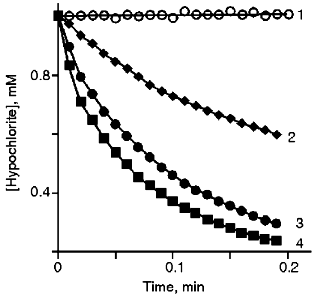
Kinetics of the Reaction of Hypochlorite with t-Butyl Hydroperoxide. It has been demonstrated [14] that HOCl/OCl- reacts with the hydroperoxide group. Representative kinetics of hypochlorite consumption in the presence of various concentrations of t-butyl hydroperoxide were measured as optical density decrease at 290 nm (OCl- absorption band) (Fig. 1). The bimolecular reaction rate constant (13 ± 2 mM-1·sec-1) was calculated using the initial parts of the curves when the reaction is pseudo-monomolecular.
Comparison of Chemiluminescence of Hypochlorite Reactions with t-Butyl Hydroperoxide and H2O2. The mechanism of the hypochlorite reaction with the hydroperoxide group is unknown. We suggested first that, similar to H2O2 (Eq. (1)), 1O2 can be formed during the reaction.Fig. 1. Kinetics of the reaction of hypochlorite with t-butyl hydroperoxide in phosphate buffer (50 mM, pH 7.4) at 20°C. t-Butyl hydroperoxide concentrations: 0 (1), 5 (2), 10 (3), 12 mM (4).
Singlet oxygen has so-called dimol emission [15]:
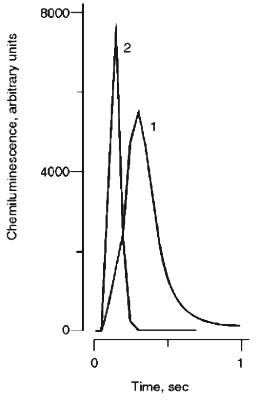
We first wanted to know whether the hypochlorite reaction with organic hydroperoxide is associated with luminescence. Kinetics of hypochlorite chemiluminescence in the visible spectral region after the addition of t-butyl hydroperoxide (curve 1) are presented in Fig. 2. The reaction is associated with an intensive chemiluminescence flash though it develops significantly slower than that obtained after the addition of H2O2 to hypochlorite (Fig. 2, curve 2).
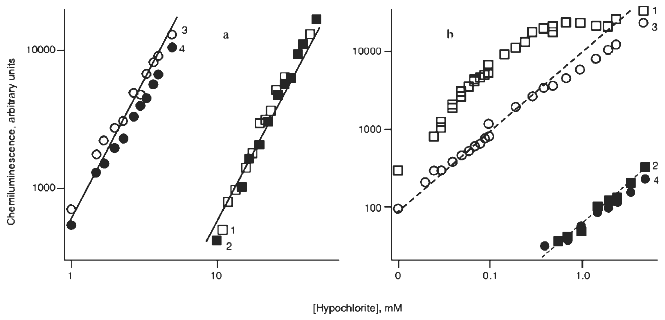
The effects of hypochlorite concentration on the integral chemiluminescence intensity in reactions with H2O2 and t-butyl hydroperoxide are presented in Figs. 3a and 3b, respectively. In the hypochlorite reaction with excess H2O2 (Fig. 3a), experimental values fit the quadratic function (indicated by continuous lines) and the red filter did not significantly decrease the luminescence intensity (curves 2 and 4, Fig. 3a). Substitution of D2O for H2O enhanced the luminescence intensity by ~10-fold (curves 3 and 4, Fig. 3a). The data are in good agreement with the literature [15, 22, 23] and unequivocally confirm the formation of 1O2 in reaction (1).Fig. 2. Kinetics of chemiluminescence (in the visible spectral region) in the systems: 1) 0.5 mM hypochlorite + 40 mM t-butyl hydroperoxide; 2) 50 mM hypochlorite + 200 mM H2O2. Incubation medium contained 100 mM carbonate buffer, pH 9.5 (37°C).
When t-butyl hydroperoxide was substituted for H2O2 (Fig. 3b), the red filter almost completely abolished the luminescence. Under these conditions, only very weak luminescence with intensity less than 1% of the initial level was measured (curves 2 and 4, Fig. 3b). The curve significantly differed from quadratic and substitution of D2O for water resulted even in a slight decrease in luminescence intensity (curve 3, Fig. 3b). The data indicate that 1O2 is not formed during the hypochlorite reaction with t-butyl hydroperoxide.Fig. 3. Influence of D2O (3, 4) and red filter (2, 4) on the effect of hypochlorite concentration on the integral chemiluminescence intensity in the visible spectral region in the systems: a) hypochlorite + 200 mM H2O2; b) hypochlorite + 40 mM t-butyl hydroperoxide. 1) Chemiluminescence in the medium prepared from H2O in the absence of the red filter. Continuous line corresponds to quadratic curve and dashed line corresponds to linear curve. Measuring conditions were the same as indicated in the legend to Fig. 2.
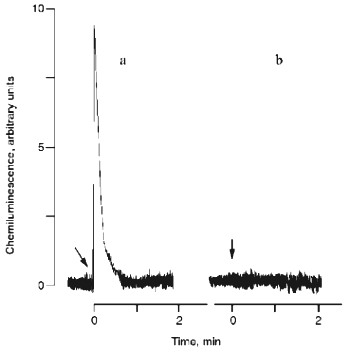
A chemiluminometer equipped with a detector based on a germanium photodiode was used for direct assay of 1O2; the apparatus can directly detect monomololecular 1O2 emission in the infrared spectral region (at 1270 nm). The data are presented in Fig. 4. As expected, hypochlorite reaction with H2O2 is associated with a chemiluminescence flash (Fig. 4a). During the hypochlorite reaction with organic hydroperoxide, monomol 1O2 emission was not detected (Fig. 4b).
1H-NMR of Products of the Reaction of Hypochlorite with t-Butyl Hydroperoxide. Thus, 1O2 is not formed in the hypochlorite-t-butyl hydroperoxide system. Then, what is causing chemiluminescence associated with the HOCl/OCl- reaction with organic hydroperoxide? At first, free radical formation during this reaction can be suggested because free radical reactions are usually associated with very weak luminescence in the visible spectral region [21].Fig. 4. Kinetics of monomol emission of singlet oxygen generated in systems: a) 10 mM hypochlorite + 20 mM H2O2; b) 10 mM hypochlorite + 20 mM t-butyl hydroperoxide. The incubation medium contained 100 mM phosphate buffer, pH 7.4 (37°C). Arrows indicate the addition of hydroperoxides.
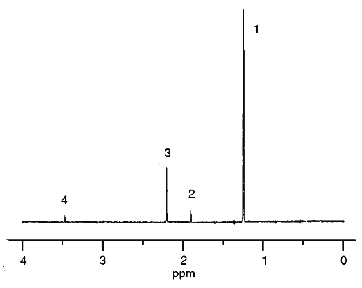
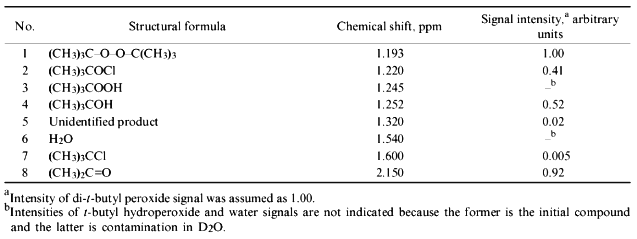
The spectrum of the products formed in the hypochlorite reaction with organic hydroperoxide (t-butyl hydroperoxide was the model compound) was studied by 1H-NMR. Hypochlorite was incubated in the presence of t-butyl hydroperoxide in medium prepared using D2O. After 10-min incubation, hydrophobic products were extracted with deuterated chloroform. The 1H-NMR spectra of D2O and CDCl3 phases were measured. The 1H-NMR spectrum of the D2O phase is shown in Fig. 5. Peaks with chemical shifts 1.90, 2.20, and 3.47 ppm correspond to methyl protons of acetate, acetone, and methanol, respectively. However, derivatives of t-butyl hydroperoxide had a single chemical shift (1.24 ppm) and thus could not be identified.
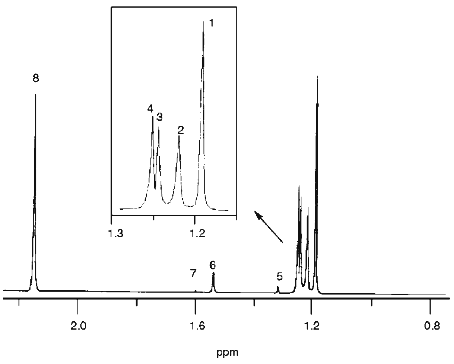
The spectrum of the CDCl3 phase is presented in Fig. 6. All major products were identified and their chemical shifts are listed in Table 1. It is important that t-butyl derivatives could be identified in the chloroform phase. Di-t-butyl peroxide (1.193 ppm), t-butyl hypochlorite (1.220 ppm), t-butanol (1.252 ppm), and acetone (2.150 ppm) are the major products.Fig. 5. 1H-NMR spectrum of hydrophilic products of the reaction of hypochlorite with t-butyl hydroperoxide in D2O medium: 1) t-butyl derivatives; 2) acetate; 3) acetone; 4) methanol. Reaction conditions and measurement of the spectrum are described under "Materials and Methods".
TABLE 1. Structural Formulae, Chemical Shifts, and Fractional Intensity of 1H-NMR Signals of the Products of Hypochlorite Reaction with t-Butyl Hydroperoxide Registered in CDCl3 PhaseFig. 6. 1H-NMR spectrum of the products of the reaction of hypochlorite with t-butyl hydroperoxide extracted with deuterated chloroform. The reaction conditions and measurement of the spectrum are described under "Materials and Methods". Numbering of the signals corresponds to the numbers of identified compounds listed in Table 1.
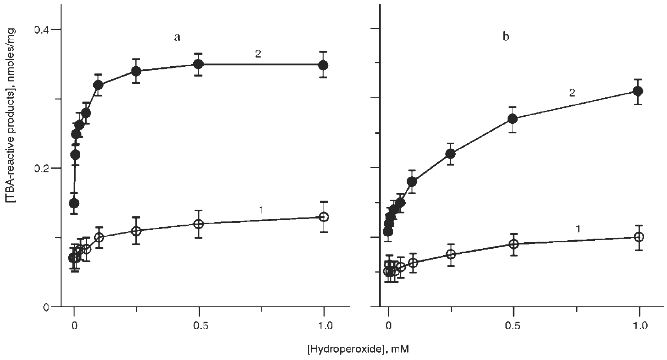
Initiation of Lipid Peroxidation in the Reaction of Hypochlorite with t-Butyl Hydroperoxide. It has been demonstrated that hypochlorite initiates the formation of LPO products in phosphatidylcholine liposomes [5, 6, 10, 11]. If the reaction of hypochlorite with t-butyl hydroperoxide results in the formation of LPO initiators, then addition of organic hydroperoxide into the incubation medium containing phosphatidylcholine liposomes and hypochlorite should enhance the accumulation of LPO products. The data of Fig. 7a (curve 1) indicate that addition of t-butyl hydroperoxide to liposomes causes some accumulation of the TBA-reactive products of LPO. If hypochlorite is added to hydroperoxide-containing liposomes, accumulation of TBA-reactive products was significantly higher (Fig. 7a, curve 2). A similar effect was observed in the presence of cumene hydroperoxide (Fig. 7b).
If 1O2 is the initiator of hypochlorite-induced LPO, then oxidation of liposomes by hypochlorite in the medium prepared using D2O should increase its lifetime, thus intensifying LPO and increasing the yield of LPO products.Fig. 7. Effect of various concentrations of t-butyl hydroperoxide (a) or cumene hydroperoxide (b) on accumulation of TBA-reactive products in phosphatidylcholine liposomes (2 mg/ml) after incubation in the absence (1) or in the presence of 50 µM hypochlorite (2). The incubation medium contained 140 mM NaCl and 10 mM phosphate buffer (pH 7.4). The incubation time was 40 min (37°C).
Hypochlorite-induced accumulation of TBA-reactive products of LPO in liposomes incubated in the media prepared using H2O and D2O is presented in Fig. 8. Substitution of D2O for H2O had no effect on liposome oxidation by hypochlorite. This suggests that 1O2 is not involved in phosphatidylcholine oxidation by hypochlorite.
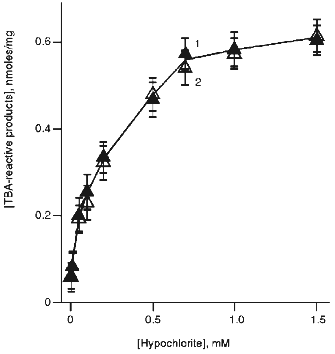
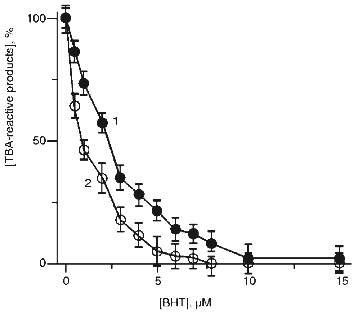
On the other hand, the data of Fig. 9 indicate that free radical scavenger, butylated hydroxytoluene (BHT), completely inhibited hypochlorite-induced accumulation of TBA-reactive products in the absence (curve 2) and in the presence of t-butyl hydroperoxide (curve 1). Thus, in both cases LPO includes the radical formation stage.Fig. 8. Effect of hypochlorite concentration on hypochlorite-induced accumulation of TBA-reactive products of LPO in phosphatidylcholine liposomes in medium prepared using H2O (curve 1) or D2O (curve 2). Incubation conditions were the same as described in the legend to Fig. 7.
Fig. 9. Effect of BHT concentration in the incubation medium on increase in TBA-reactive products of LPO in phosphatidylcholine liposomes (2 mg/ml) after incubation with hypochlorite (initial concentration 50 µM) in the presence (1) or in the absence of 50 µM t-butyl hydroperoxide (2). Increase in TBA-reactive products in the absence of BHT is taken as 100%. The incubation medium contained 140 mM NaCl and 10 mM phosphate buffer (pH 7.4). The incubation time was 40 min (37°C).
DISCUSSION
Hypochlorite is a strong oxidizer and is synthesized in the reaction of hydrogen peroxide with chloride:
Indeed, the data indicate that hypochlorite reacts with t-butyl hydroperoxide with rate constant 13 ± 2 mM-1·sec-1. The reaction is associated with very weak luminescence, chemiluminescence, but its mechanism is unknown. At least two possibilities can be suggested. First, similar to H2O2, the reaction may occur according to Eq. (1) with the formation of 1O2 that is known as an LPO initiator [16, 24]. Second, free radical intermediates may be synthesized during the reaction and they also usually initiate LPO [21, 24].
The data of the present study indicate that 1O2 is not formed during the reaction of hypochlorite with organic hydroperoxide. The following results support this conclusion.
1. Effect of hypochlorite concentration on the integral chemiluminescence intensity in the visible spectral region was not quadratic (Fig. 3b, curve 1) as it should be according to Eq. (2) in case of reaction (1) (Fig. 3a).
2. A red filter transmitting the light >600 nm almost completely abolished chemiluminescence associated with the hypochlorite reaction with t-butyl hydroperoxide (Fig. 3b, curves 2 and 4) and did not affect that in the hypochlorite reaction with H2O2 (Fig. 3a, curves 2 and 4). Thus, luminescence registered during the reaction of hypochlorite with t-butyl hydroperoxide is outside the red spectral region characteristic for luminescence of singlet oxygen [15].
3. Substitution of D2O for H2O increases the lifetime of 1O2 and intensity of its luminescence by ~10-fold. This is evident during the reaction of hypochlorite with H2O2 (Fig. 3a, curves 3 and 4). However, during the reaction of hypochlorite with t-butyl hydroperoxide such substitution did not enhance illumination intensity (Fig. 3b, curves 3 and 4).
4. If accumulation of LPO products in liposomes in the presence of hypochlorite, which was observed previously [10, 11] and in the present work (Figs. 7 and 8), is due to 1O2 synthesis, then the effect should be enhanced in medium prepared using D2O. However, hypochlorite incubation with liposomes in D2O medium did not increase the accumulation of LPO products (Fig. 8).
5. Finally, we were unable to register monomol emission of 1O2 in the infrared region after addition of t-butyl hydroperoxide (unlike H2O2) to hypochlorite (Fig. 4, a and b).
The data exclude 1O2 synthesis in the reaction of hypochlorite with organic hydroperoxide. Thus, the reaction should involve the formation of free radicals.
This hypothesis is supported by the following data. First, the reaction of hypochlorite with organic hydroperoxide in the solution (Figs. 2 and 3b) or incorporated into dimyristoylphosphatidylcholine liposomes [14] is associated with chemiluminescence outside the red spectral region that is characteristic for free radical reactions [21, 24]. Second, the stoichiometric ratio hypochlorite:hydroperoxide significantly exceeds equimolar [14], whereas reaction (1) is equimolar when free radical intermediates do not participate [25]. Finally, the data of Fig. 9 indicate that accumulation of TBA-reactive products in egg phosphatidylcholine liposomes containing t-butyl hydroperoxide in the presence of hypochlorite was suppressed by the free radical scavenger BHT.
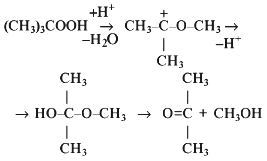
The spectrum of products formed in the reaction of hypochlorite with organic hydroperoxide (t-butyl hydroperoxide was the model compound) was studied by 1H-NMR. The spectra presented in Figs. 5 and 6 and Table 1 indicate that di-t-butyl peroxide, t-butyl hypochlorite, t-butanol, acetone, acetate, and methanol are the major reaction products. The three latter products can be formed during the Hock reaction [26]:
with subsequent haloformic reaction [26] according to the total equation:
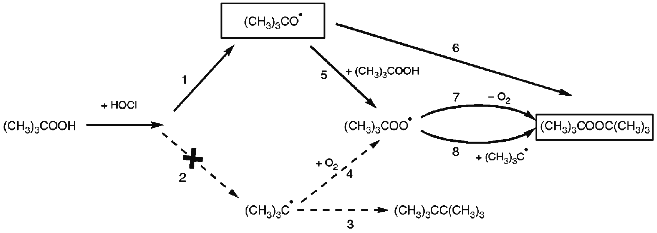
The major reaction product di-t-butyl peroxide is of particular importance. It does not react with hypochlorite [14], is the final product, and can be formed via radical stage only according to our opinion, i.e., via alkoxyl or alkyl radical as indicated in Fig. 10 (pathways 1 and 2, respectively).
Assume that alkyl (t-butyl) radical is the intermediate. Then, first, hexamethylethane (pathway 2-3 in Fig. 10) should be detected among the products due to the recombination of two t-butyl radicals; its 1H-NMR signal has chemical shift 0.85 ppm as indicated by a separate experiment. Second, the yield of di-t-butyl peroxide should depend on oxygen concentration in the reaction medium (see pathway 2-4-8 in Fig. 10). Both characteristics were not confirmed experimentally. The peak with chemical shift in the region 0.85 ppm is absent from the 1H-NMR spectrum (Fig. 6). Varying O2 concentration in the reaction medium by bubbling with argon did not influence the yield of di-t-butyl peroxide (data not shown). Evidently, pathway 2 (Fig. 10) does not occur in fact. Thus, alkoxyl (t-butoxyl) radical is the free radical intermediate in the hypochlorite reaction with organic hydroperoxide and the recombination of two such radicals results in the formation of di-t-butyl peroxide (pathway 1-6 in Fig. 10).Fig. 10. Hypothetical scheme of t-butoxyl radical (CH3)3CO· participation in the reaction of hypochlorite with t-butyl hydroperoxide. For details see the text.
Formation of another reaction product, t-butanol, can be easily explained via t-butoxyl radical, i.e., by the following reaction (pathway 1-5 in Fig. 10):
Finally, the last of the major reaction products, t-butyl hypochlorite, can be form by direct interaction of hypochlorite with t-butyl hydroperoxide or t-butanol [17] and by the reaction of t-butoxyl radical with HOCl or Cl2. The latter two species are in equilibrium in aqueous solution [29]:
(CH3)3C-O· + HOCl --> (CH3)3C-OCl + ·OH ,
(CH3)3C-O· + Cl2 --> (CH3)3C-OCl + Cl· .
Nevertheless, the data suggest that the reaction of hypochlorite with organic hydroperoxide involves a radical formation stage. Alkoxyl radical, which may initiate free radical LPO reactions [21], is the most probable intermediate.
If this is true then hydroperoxide groups present in the lipid should promote the development of hypochlorite-induced LPO. Indeed, the data of Fig. 7 (a and b) indicate that addition of organic hydroperoxides in the incubation medium containing phosphatidylcholine liposomes and HOCl/OCl- activates LPO and enhances the accumulation of TBA-reactive products. The effect was detected at very low concentrations of hydroperoxide and was completely abolished by the radical scavenger BHT (Fig. 9). Addition of 2.5 nmoles of t-butyl hydroperoxide per mg phospholipid increases the accumulation of TBA-reactive products in the incubation medium containing HOCl/OCl- and phosphatidylcholine liposomes by ~2-fold versus the accumulation in the absence of hydroperoxide. It is important that this hydroperoxide concentration is comparable to that in vivo. For example, according to [24], 4.8 ± 0.4 nmoles of hydroperoxides per mg lipid are present in human serum. The contents of organic hydroperoxides in tissues of various animals registered by amperometric titration and polarography varied from 0.4 ± 0.1 to 19.6 ± 1.1 nmoles per mg lipid [24].
Thus, the data of the present study indicate that hypochlorite reacts with organic hydroperoxide without the formation of singlet oxygen. The reaction includes free radical stages evidently via alkoxyl radicals, is associated with chemiluminescence, and induces accumulation of LPO products in phosphatidylcholine liposomes. This suggests that organic hydroperoxides, which are always present in some amount in the lipid phase of biological membranes and lipoproteins in vivo, can be the same compounds whose interaction with hypochlorite results in the formation of the free radical source thus initiating LPO.
The authors are indebted to H. Sies and K. Briviba (Institute of Physiological Chemistry I, Dusseldorf University, Germany) for their help in the measurement of the monomol emission of singlet oxygen. The participation of O. M. Panasenko in the study was supported by the Alexander Humboldt Foundation (Germany).
LITERATURE CITED
1.Panasenko, O. M., and Sergienko, V. I. (1993)
Biol. Membr. (Moscow), 10, 341-382.
2.Berliner, J. A., and Heinecke, J. W. (1996) Free
Radic. Biol. Med., 20, 707-727.
3.Edwards, S. W. (1994) Biochemistry and
Physiology of the Neutrophil, University Press, Cambridge.
4.Panasenko, O. M., Evgina, S. A., Aidyraliev, R. K.,
Sergienko, V. I., and Vladimirov, Yu. A. (1994) Free Radic. Biol.
Med., 16, 143-148.
5.Panasenko, O. M., Evgina, S. A., Driomina, E. S.,
Sharov, V. S., Sergienko, V. I., and Vladimirov, Yu. A. (1994) Free
Radic. Biol. Med., 19, 133-140.
6.Panasenko, O. M., Evgina, S. A., Driomina, E. S.,
Sharov, V. S., Sergienko, V. I., and Vladimirov, Yu. A. (1994) Free
Radic. Biol. Med., 16, 14.
7.Evgina, S. A., Panasenko, O. M., Sergienko, V. I.,
and Vladimirov, Yu. A. (1992) Biol. Membr. (Moscow), 9,
946-953.
8.Panasenko, O. M., Evgina, S. A., Driomina, E. S.,
Sharov, V. S., Sergienko, V. I., and Vladimirov, Yu. A. (1995) Biol.
Membr. (Moscow), 12, 191-199.
9.Hazell, L. J., and Stocker, R. (1993) Biochem.
J., 290, 165-172.
10.Panasenko, O. M., Arnhold, J., Schiller, J.,
Arnold, K., and Sergienko, V. I. (1994) Biochim. Biophys. Acta,
1215, 259-266.
11.Panasenko, O. M., and Arnhold, J. (1996) Biol.
Membr. (Moscow), 13, 89-99.
12.Arnhold, J., Panasenko, O. M., Schiller, J.,
Vladimirov, Yu. A., and Arnold, K. (1995) Chem. Phys. Lipids,
78, 55-64.
13.Winterbourn, C. C., van der Berg, J. J. M.,
Roitman, E., and Kuypers, F. A. (1992) Arch. Biochem. Biophys.,
296, 547-555.
14.Panasenko, O. M., Arnhold, J., Vladimirov, Yu.
A., and Sergienko, V. I. (1995) Biochemistry (Moscow),
60, 1419-1428 (Russ.).
15.Kanofsky, J. R. (1989) Chem.-Biol.
Interact., 70, 1-28.
16.Thomas, M. J., and Pryor, W. A. (1980)
Lipids, 15, 544-548.
17.Metzger, J. O. (1989) in Houben-Weyl. Methoden
fuer Organischen Chemie, 4 Auflage, Bd. E19a, C-Radikale,
Georg-Thieme Verlag, Stuttgart, New York, pp. 60-145.
18.Morris, J. C. (1966) J. Phys. Chem.,
70, 3798-3805.
19.Berrs, R. F., and Sizer, I. W. (1952) J. Biol.
Chem., 195, 133-140.
20.Di Mascio, P., Devasagayam, T. P. A., Kaiser, S.,
and Sies, H. (1992) Meth. Enzymol., 213, 429-438.
21.Vladimirov, Yu. A., and Archakov, A. I. (1972)
Lipid Peroxidation in Biological Membranes [in Russian], Nauka,
Moscow.
22.Merkel, P. B., Nilsson, R., and Kearns, D. R.
(1972) J. Am. Chem. Soc., 94, 1030-1031.
23.Rodgers, M. A. J., and Snowden, P. T. (1982)
J. Am. Chem. Soc., 104, 5541-5543.
24.Kagan, V. E., Orlov, O. N., and Prilipko, L. L.
(1986) Advances in Science and Technology, Biophysics [in
Russian], Vol. 18, VINITI, Moscow, pp. 5-135.
25.Held, A. M., Halko, D. J., and Hurst, J. K.
(1978) J. Am. Chem. Soc., 100, 5732-5740.
26.Becker, H. G. O., Domschke, G., Fanghaenel, E.,
Eischer, K. G., Mayer, R., Pavel, D., Schmidt, H., and Schwetlick, K.
(1988) Organikum, VEB Deutscher Verlag der Wissenschaften,
Berlin, pp. 375-578.
27.Pryor, W. A. (1986) Ann. Rev. Physiol.,
48, 657-667.
28.Howard, J. A., and Ingold, K. U. (1968) J. Am.
Chem. Soc., 90, 1056-1058.
29.Eigen, M., and Kustin, K. (1962) J. Am. Chem.
Soc., 84, 1355-1361.