Ultrastructure of Mitochondrial Reticulum of Human Cardiomyocytes in Alcohol Cardiomyopathy
Yu. V. Sudarikova,1 L. E. Bakeeva,1,2 and V. G. Tsiplenkova3
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3181; E-mail: yuli@energ.genebee.msu.su2To whom correspondence should be addressed.
3Cardiology Research Center, Russian Academy of Medical Sciences, ul. 3-ya Cherepkovskaya 15a, Moscow, 122551 Russia; fax: (095) 414-6699.
Submitted March 17, 1997.
Endomyocardial left ventricular biopsy material from patients with chronic alcoholism exhibits structural alterations of the mitochondrial reticulum in alcohol cardiomyopathy. The progress of gradually developing structural changes depends on the stage of the disease. The early stage of the disease is characterized by spatial reorganization of the mitochondrial reticulum: intermitochondrial contacts disappear and mitochondria form separate clusters uniformly distributed within a muscle cell. Subsequently, in the second and third stages of chronic disease, destructive irreversible changes in the ultrastructural organization of mitochondria develop. Megamitochondria and septate mitochondria appear. A third additional compartment containing granules forms in mitochondria. Many lipofuscin granules appear due to the accumulation of lipids in mitochondria. Structural changes of the mitochondrial reticulum are considered as a compensatory adaptive reaction of the cardiomyocyte mitochondrial system in response to altered myocardial function in alcohol cardiomyopathy, including abnormalities in the cardiac rhythm and ventricular conductance.
KEY WORDS: mitochondrial reticulum, ultrastructure, intermitochondrial contacts, alcohol cardiomyopathy.
Striated muscle mitochondria of vertebrates represent a single structure, the mitochondrial reticulum, where the whole population of mitochondria joins into a single system via special membrane contact structures, intermitochondrial contacts. These mitochondrial contacts are suggested to have a system-forming function organizing mitochondria into a single structure-functional system which covers the whole muscle cell [1].
We have shown previously that the mitochondrial reticulum is also present in human cardiomyocytes. It has the same structural organization which is typical for the cardiomyocytes of vertebrates [2]. In human cardiomyocytes numerous weakly branched mitochondria are located along myofibrils. They are pooled by intermitochondrial contacts into united layers surrounding myofibrils. Mitochondria of perinuclear accumulations are connected with each other and also with mitochondria located around myofibrils. Thus, a network of mitochondrial membranes, the mitochondrial reticulum, covers the whole space of a muscular cell constituting a membrane basis of myocardial cell ultrastructure.
The evaluation of the biological significance of such lengthy membrane systems of the mitochondrial reticulum in a cell is an important problem. Does this structure have a special functional role, or does it just represent mitochondrial localization characteristic for muscle tissue? Are mitochondrial contacts system forming elements (like intercellular contacts) or is this a simple mechanical adhesion, a fold of the mitochondrial membranes resulting from close orientation of the organelles?
Possibilities for using artificial model systems for clarification of these queries are limited. We believe that studies of the real processes occurring in vivo, especially under pathological conditions, have priority. Thus, detailed investigation of the myocardial reticulum ultrastructure in human cardiomyocytes at various stages of alcohol cardiomyopathy was the main goal of this study.
MATERIALS AND METHODS
Endomyocardial left ventricular biopsy material was taken during coronary angiography and ventriculography of four patients of age 26-39 years. They were initially cured at an addiction hospital with diagnosis of chronic alcoholism of the second and third stages. However, due to abnormalities in their cardiac rhythms (paroxysmal tachycardia, ciliary and ventricular arrhythmias) or ventricular conductance without symptoms of blood circulation insufficiency and subjective complaints they were transferred to the Cardiology Research Center. Using clinical data and results of analysis of endomyocardial bioptates, alcohol cardiomyopathy was diagnosed.
Bioptates from the left ventricular myocardium (2-3 pieces) were fixed with 2.5% glutaraldehyde in phosphate buffer, then postfixed with osmic acid and after dehydration in increasing alcohol concentrations they were fixed in epoxy resin. Ultrathin serial slices were prepared using an LKB-V ultramicrotome (Sweden), contrasted by uranyl acetate and lead citrate, and then examined in a JEM-100CX Hitachi-12 electron microscope (Japan) under accelerating voltage of 80 and 75 kV, respectively.
RESULTS
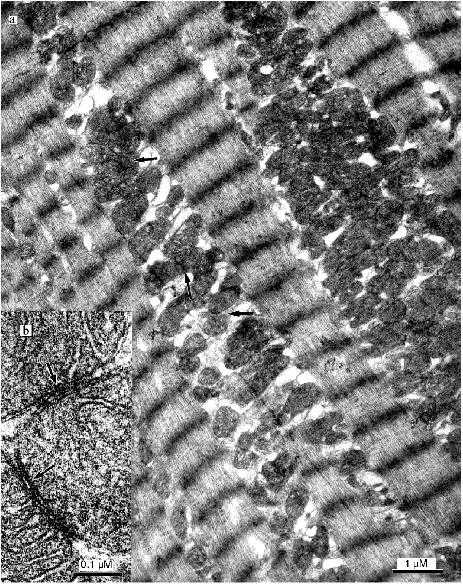
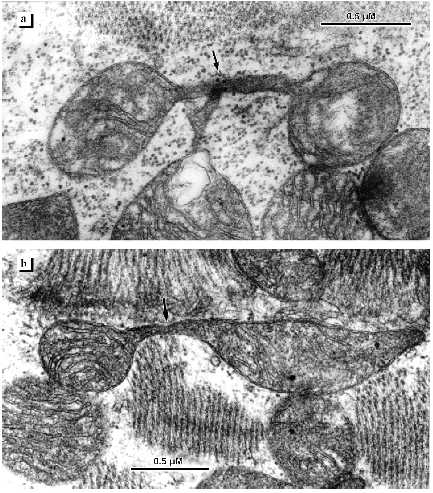
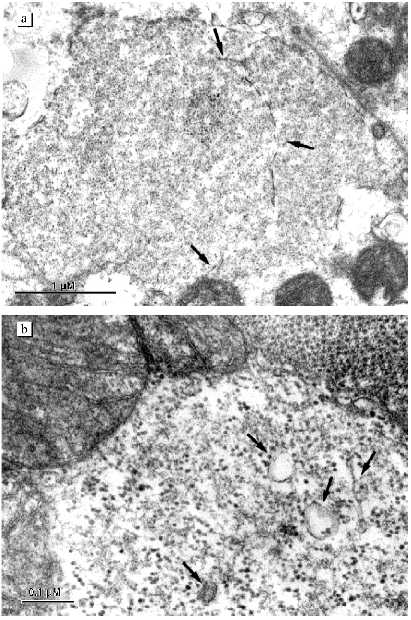
Figure 1a shows an electron micrograph of an ultrathin longitudinal slice of a left ventricular cardiomyocyte from a 26 year old patient with signs of the initial stage of cardiomyopathy. Layers of small mitochondria are located along myofibrils. In these layers mitochondria are connected by contact structures. Under low magnifications the contact structures look like electron-dense lines between neighboring mitochondria. Greater magnifications demonstrate that this is a specially organized intermembrane contact structure with characteristic morphological signs: compact border, confluence of the outer mitochondrial membranes of contacting mitochondria, significant increase in electron density of the outer and inner mitochondrial membranes, condensation of electron-dense substance of the intermembrane space in the contact zone (Fig. 1b). Thus, at the initial stage of alcohol cardiomyopathy the ultrastructure of cardiomyocytes corresponds to generally accepted ideas about the organization of cardiomyocyte mitochondria as a united mitochondrial system. Changes or abnormalities in the structure were not found.
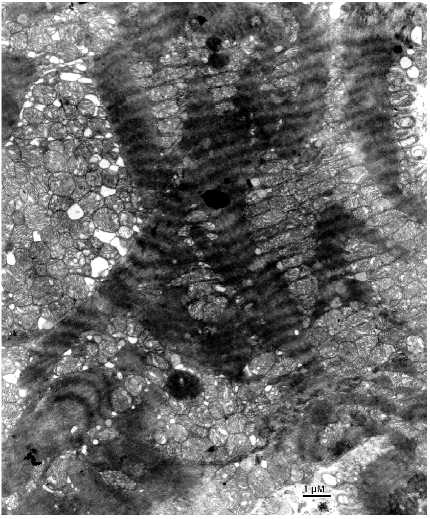
Subsequent development of the disease is accompanied by significant changes in the ultrastructure of the cardiomyocyte mitochondrial system. Figures 2-4 demonstrate the ultrastructures of left ventricular cardiomyocyte mitochondria of patients with ages ranging from 34 to 39 years who were suffering from alcoholism for a long time.Fig. 1. Ultrastructure of left ventricle cardiomyocyte of a patient with the initial stage of alcohol cardiomyopathy (arrows show intermitochondrial contacts): a) general view; b) intermitochondrial contacts.
Fig. 2. Ultrastructure of a left ventricle cardiomyocyte of a patient with a long history of alcoholism.
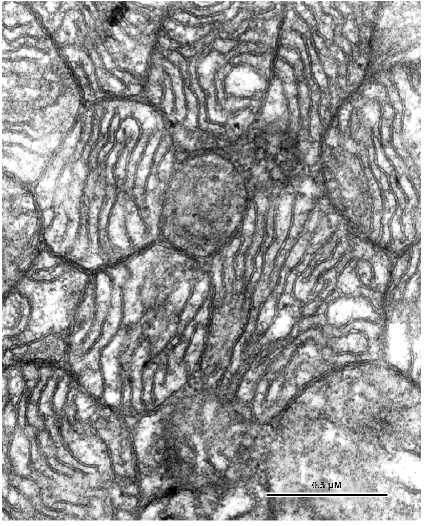
Fig. 3. Ultrastructure of a left ventricle cardiomyocyte mitochondrial cluster of a patient with a long history of alcoholism. In spite of compact packing of mitochondria, intermitochondrial contacts are absent.
Marked changes in the arrangement of the internal mitochondrial organization were not found. There was a slight enlightenment of the mitochondrial matrix. However, the main mitochondrial parameters: 1) volume ratio of matrix and intermembrane space; 2) configuration of the inner mitochondrial membrane, forming numerous correctly oriented cristae correspond to the ultrastructure of intact cardiomyocyte mitochondria. In contrast to relatively unchanged internal ultrastructure a major part of the mitochondrial spatial organization of the mitochondrial population of cardiomyocyte is changed at this stage of the disease. However, it is impossible to describe a universal stereotype character of the changes in the mitochondrial system because its structure is characterized by a significant heterogeneity. Even within one slice, altered cells neighbor cells without changes in the ultrastructure of the mitochondrial system. Figure 2 shows a cardiomyocyte where the topography of mitochondria does not correspond to that typical for myocardial muscle. Intermyofibrillar mitochondria form a few dense clusters and mitochondria from the subsarcolemmal population are strongly hypertrophied and also form a large cluster. Under low magnifications mitochondria in these clusters seem to maintain intermitochondrial contacts because they are tightly bound and their borders look like electron dense lines. However, under high magnifications morphological signs typical for intermitochondrial contacts were not found in spite of dense packing of mitochondria in these clusters (Fig. 3). Confluence of the outer mitochondrial membranes was not found; four separated mitochondrial membranes of contacting mitochondria can be clearly recognized. Increased electron density of the intermembrane space and the mitochondrial membranes is absent. Thus, increased electron density at the sites of tight contacts of mitochondria seen under low magnifications is in fact the result of close orientation of mitochondrial membranes. These data are an important argument that intermitochondrial contacts represent a special intermembrane structure rather than the result of tight contact between mitochondrial membranes or mechanical compression of the tissue during myofibril contraction. Such dramatically altered cardiomyocytes neighbor with cells possessing structure that corresponds to the generally accepted ideas about cardiomyocyte structure. Figure 4 shows the ultrastructure of such a cardiomyocyte. Mitochondria form vertical rows along myofibrils and they look as if they were connected by intermitochondrial contacts. However, analysis of the ultrastructure of serial slices revealed that intermitochondrial contacts are absent in these cells. Thus, the structure of the mitochondrial reticulum, a united mitochondrial system situated among myofibrils is rearranged in cardiomyocytes of these patients.Fig. 4. Part of a left ventricle cardiomyocyte with unchanged ultrastructure from of a patient with alcohol cardiomyopathy.
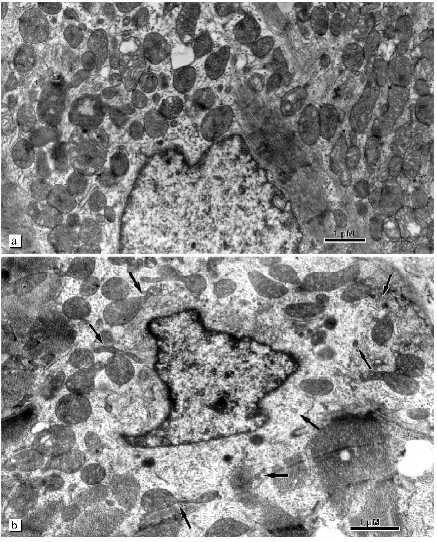
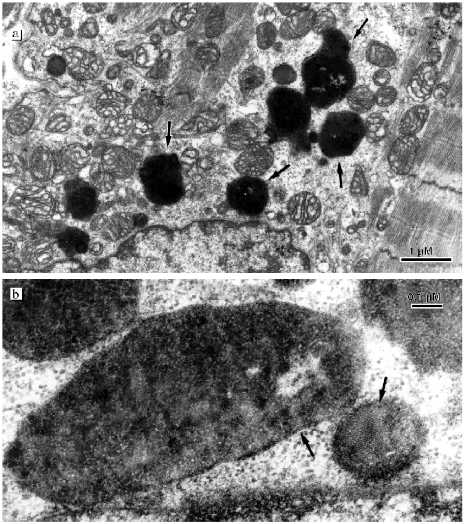
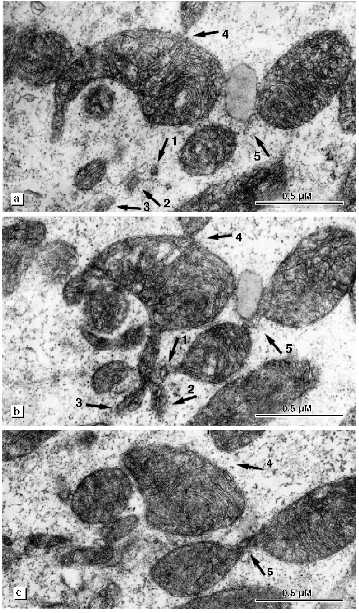
The changes of the mitochondrial system is also extended to the perinuclear zone. Assembly of large mitochondria around the nucleus known in the literature as mitochondrial "caps" is absent. Figure 5a shows perinuclear accumulation of mitochondria at the initial stage of alcohol cardiomyopathy. Subsequent development of the disease is characterized by appearance of a light zone around the nucleus that lacks myofibrils and mitochondria (Fig. 5b). Separated mitochondria are located on the periphery of this zone. It looks as if the mitochondria are repelled by the nucleus. Such zones have a great number of large electron-dense structures of oval form containing small particles with high electron density (Fig. 6a). They are known in the literature as lipofuscin granules. The origin and mechanism of formation of these particles remain unclear. We found that in our preparations these structures are limited by two membranes and they may contain crystal-like structures, yolk plates. Mitochondria are considered as a typical place for their localization (Fig. 6b). The existence of such morphological signs suggests that these structures may represent mitochondria where lipofuscin accumulates in the form of electron dense particles and fills their entire internal space. Three-dimensional reconstruction revealed that the perinuclear zone of the mitochondria possess an unusual morphology. There are many small mitochondria with few cristae or roundish or filiform double membrane structures. Larger roundish mitochondria have a structure typical for cardiomyocytes (Fig. 5b). In reality, the perinuclear zone mitochondria possess either dumbbell-like form with a cross-piece of various thickness or they form thin lengthy double-membrane outgrowths often of odd form. In these cross-pieces or outgrowths constriction zones are well recognized and they are always present (Fig. 7, a and b). Thus, the population of small mitochondria in the perinuclear zone that might be identified on single slices as promitochondria (by size and internal organization) represents in reality cross-sections of large mitochondrial outgrowths. Taking into consideration the literature data this unusual mitochondrial ultrastructure can be explained as the electron microscopy picture of reproduction, renewal of the mitochondrial population. It is logical to suggest that the renewal process can take place because destructive processes are accompanied by irreversible changes in some of the mitochondria. However, we do not have enough data for such a conclusion because in serial slices we did not find separately located small mitochondria. We reconstructed 15 sites of slices that contain such structures and in all cases small mitochondria represent cross-sections of elongated outgrowths of large mitochondria (Fig. 8, a-c).
Fig. 5. Ultrastructure of perinuclear zone mitochondria of cardiomyocytes from patients with alcohol cardiomyopathy: a) perinuclear zone of left ventricle cardiomyocyte of a patient with initial signs of alcohol cardiomyopathy (assemblies of numerous mitochondria around the nucleus can be seen); b) perinuclear zone of left ventricle cardiomyocyte of a patient with developed alcohol cardiomyopathy (arrows show cross-sections of small mitochondria, mitochondrial outgrowths, and constrictions).
Fig. 6. Ultrastructure of lipofuscin granules in cardiomyocytes of patients with alcohol cardiomyopathy (arrows show two limiting membranes and a yolk plate): a) accumulation of lipofuscin granules in the perinuclear zone; b) lipofuscin granules under high magnification.
Fig. 7. Ultrastructure of perinuclear zone mitochondria of cardiomyocytes from patients with alcohol cardiomyopathy (a, b). Arrows show constrictions of mitochondria.
Unusual structures were found in the perinuclear zone and also among myofibrils (Figs. 9 and 10). The sizes of these structures significantly exceeded those of surrounding mitochondria. They are limited by two membranes and their internal space is packed with granules of two types and of fibrous elements. Serial slices demonstrate that the sizes of these structures are comparable to the nucleus and some regions of the matrix of these structures contain closed membrane structures whose morphological parameters might correspond to mitochondrial cristae (Fig. 10, a and b). These structures were described previously by one of us as giant vacuoles which are a characteristic sign of alcohol ultrastructure in alcoholic heart disease [3]. Some authors consider the appearance of these structures in cardiomyocytes as a partial necrosis event [4].Fig. 8. Ultrastructure of perinuclear zone mitochondria of left ventricular cardiomyocyte of a patient with developed alcohol cardiomyopathy: a-c) serial slices of mitochondrial groups. Separate small mitochondria indicated by arrows 1, 2, and 3 are cross-sections of elongated outgrowths of large mitochondria. Arrows 4 and 5 show constrictions of mitochondria detected in the serial slices.
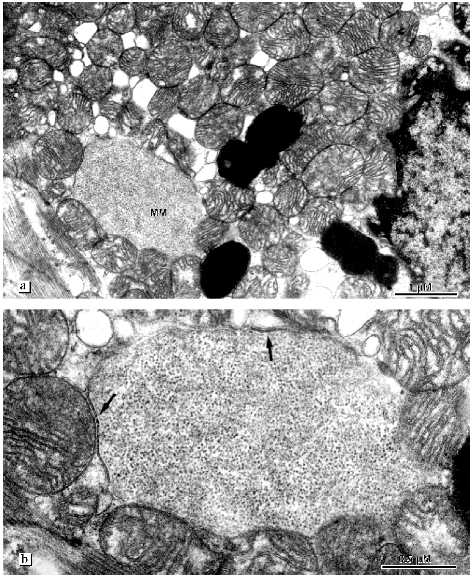
Fig. 9. Peculiarities of left ventricle cardiomyocyte mitochondrial structures in alcohol cardiomyopathy in patients with a long history of alcoholism. Ultrastructure of megamitochondria: a) general view indicating distribution of megamitochondria (MM) in the mitochondrial cluster; b) the same megamitochondrion under high magnification (arrows show two limiting membranes).
The most pronounced changes in cardiomyocyte ultrastructure were found in a 39 year old patient suffering from alcohol cardiomyopathy for a longer period compared with the other patients. Destructive changes of mitochondria progress. The number of lipofuscin granules increased. They are located as chains along myofibrils at places characteristic of localization of mitochondria. The internal organization of most mitochondria are changed. Inside the mitochondria a closed space limited by a membrane appears. This space contains inclusions similar to lipid drops. Serial slices revealed that this formation spans the whole internal mitochondrial space. Only the two first and two last slices did not reveal this structure (Fig. 11, a-f). Thus, besides intermembrane space and matrix a third additional compartment appears in mitochondria without changes of the total volume. In the mitochondrial population septate and multicamerate mitochondria appear. Two or three separate chambers each of which is limited by the inner mitochondrial membrane are settled under one outer mitochondrial membrane. Cardiomyocyte mitochondria are located separately. Intermitochondrial contacts and structures mimicking these contacts under low magnifications are absent. Mitochondria are accumulated around fat drops and mitochondria closely bound to them. Mitochondrial sites that contact with fat drops are characterized by electron-dense zones formed by the confluence or tight binding of mitochondrial membranes.Fig. 10. Ultrastructure of megamitochondria of left ventricle cardiomyocytes in alcohol cardiomyopathy in patients with a long history of alcoholism: a, b) elements of the internal organization of megamitochondria. Arrows indicate closed membrane structures.
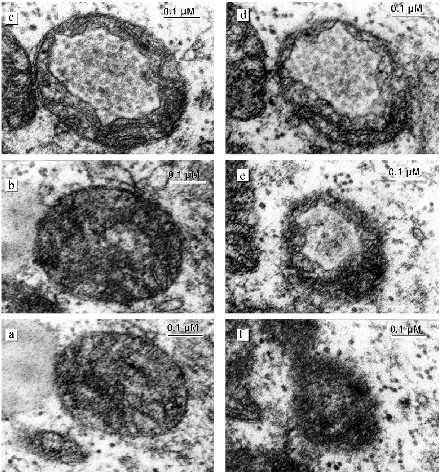
Fig. 11. Change in mitochondrial structure of left ventricle cardiomyocytes in alcohol cardiomyopathy in patients with a long history of alcoholism. A series of 16 slices through a whole separate mitochondrion which contains a third additional compartment filled with inclusions was prepared. The additional compartment was situated from the 3rd to the 14th slices inclusively. The following slices are shown: a) 3rd; b) 4th; c) 6th; d) 8th; e) 14th; f) 15th.
DISCUSSION
Skulachev's hypothesis of possible energy transmission in a cell along lengthy membrane structures is well known now and good experimental evidence supports a prediction about the functional role of the mitochondrial membrane as an "intracellular cable" [5, 6]. Skulachev also suggested that another function of mitochondrial reticulum membranes can consist of lateral transport of respiratory substrates and molecular oxygen. Fatty acids are the main oxidizing substrate in muscle tissue. It may be suggested that fatty acids formed inside cells during lipolysis of fat drops reach places of their oxidation without dilution in the cytosol. It can actually be seen that the contact between mitochondria and fatty acids is so tight that mitochondrial membranes flow together into a single electron-dense line, and it is difficult to distinguish them from matrix space. This phenomenon has been described in the literature also by other authors. According to Skulachev's view the mitochondrial reticulum can also resolve a problem of intracellular transport of molecular oxygen. Our studies show that alcohol cardiomyopathy is accompanied by significant changes in the structure of the mitochondrial reticulum and consequently its functioning as a united system. The progress of changes of the mitochondrial reticulum slowly develops and depends on a stage of the disease. Initially, spatial reorganization of the mitochondrial reticulum occurs. It is characterized by disappearance of intermitochondrial contacts and the mitochondrial population regroups into a few clusters uniformly distributed over the whole muscle cell. Appearance of mitochondrial clusters is one widespread and widely studied phenomena of ultrastructural reorganization of mitochondrial membrane systems of both plant and animal cells. This is an adaptive compensatory reaction of the mitochondrial system of the cell to changes in the physiological state of the organism (influences of cold or weak illumination for plants or hibernation for animals) [7]. Simultaneous biochemical and electron microscopy studies revealed formation of mitochondrial clusters in liver cells of hibernating hamsters that occurs in conditions of decreased respiration and ATP synthesis and deenergization of mitochondria. Skulachev suggests that uncoupling of oxidative phosphorylation in these conditions is stipulated by the presence of Ca2+-dependent cyclosporin-sensitive pore of the inner mitochondrial membrane and so cyclosporin A can prevent the formation of mitochondrial clusters [6]. Using pieces of isolated liver, cyclosporin A was demonstrated to prevent the formation of mitochondrial clusters [8]. Appearance of Ca2+-dependent cyclosporin-sensitive pore has recently been reported in cardiomyocytes during chemical anoxia [9]. Thus, the formation of mitochondrial clusters can be considered as an adaptation of the mitochondrial system to changes in the metabolic activity under stress or various physiological conditions of an organism. Our studies demonstrate that intermitochondrial contacts are the most sensitive to changes of the physiological state of the organism's structural elements of the mitochondrial reticulum. Previously one of us showed that in sympathectomized rats myocardial hypertrophy with depression of cardiac contractility caused by impairments of mitochondrial energetics is characterized by the absence of intermitochondrial contacts in cardiomyocytes [10]. Studies of various types of cardiac pathology revealed changes in the number of mitochondrial contacts [11]. This supports Skulachev's hypothesis about one possible function of intermitochondrial contacts as structures providing elimination of damaged regions of the mitochondrial reticulum [5]. In patients with ischemic heart disease with insufficiency of blood circulation partial changes in intermitochondrial contact structure are possible [2]. In later stages of the disease, when resources of the cardiomyocyte mitochondrial system for normalization of energy metabolism are obviously exhausted, destructive irreversible changes in the myocardial ultrastructure occur. Megamitochondria and septate mitochondria appear. The main population of mitochondria is characterized by reduction of the matrix volume due to the appearance in the matrix space of an additional third compartment containing inclusions. The appearance of a special type of mitochondria, megamitochondria, was described in the literature for various diseases or changes of physiological conditions of the organism [1]. Megamitochondria are found in a tissue of diseased organ. They sharply differ from the main mitochondrial population by sizes and unusual internal organization. These aberrant mitochondria are often considered as "pathological abnormality" or as process of mitochondrial degeneration. A notion also exists that the appearance of such mitochondria may be stipulated by energy requirements of cellular metabolism under various pathological processes. Data of animals experiments suggest impairments in the energetics and pathology of oxidative phosphorylation under alcohol intoxication. Twofold decrease of cytochrome oxidase concentration and activity was found in baboons which received ethanol [12]. Ethanol drinking in rats causes a decrease of oxidative phosphorylation efficiency and weakening of factor F1 connection with mitochondrial membrane [13].
Thus, alcohol cardiomyopathy is characterized by significant changes in the structure of the mitochondrial reticulum, a united mitochondrial system of cardiomyocytes. These changes can be defined as structural rearrangement of the mitochondrial reticulum. The process of this rearrangement gradually develops depending on the stage of the disease. First of all, spatial reorganization of the mitochondrial reticulum occurs: intermitochondrial contacts disappear, the mitochondrial population regroups into separate clusters uniformly distributed over the space of a muscle cell. Subsequently, changes in the internal organization of the mitochondria occur. Megamitochondria and septate mitochondria appear. In most mitochondria a third compartment is formed which results in a reduction of the matrix volume. The initial changes of the mitochondrial system are reversible, whereas subsequent changes in the internal mitochondrial organization are destructive and irreversible. Changes in the structure of the mitochondrial reticulum found in the present study demonstrate that functions of the mitochondrial system in alcohol cardiomyopathy are limited. These changes in the ultrastructure of the mitochondrial reticulum may be considered as a sign of impairments of myocardial functioning.
The authors thank V. P. Skulachev for scientific consultation during this study.
This work was supported by the Russian Foundation for Basic Research (grant No. 97-04-49149).
LITERATURE CITED
1.Bakeeva, L. E., and Chentsov, Yu. S. (1989)
Advances in Science and Technology. General Biology [in
Russian], Vol. 9, VINITI, Moscow, pp. 1-104.
2.Bakeeva, L. E., Tsiplenkova, V. G., and
Beskrovnova, N. N. (1996) Arkh. Patol., 58, 49-54.
3.Tsiplenkova, V. G., Vihert, A. M., and
Cherpachenko, N. M. (1986) J. Amer. Coll. Cardiol., 8,
22A-32A.
4.Avtsyn, A. P., and Shakhlamov, V. A. (1979)
Ultrastructural Basis of Cell Pathology [in Russian], Meditsina,
Moscow.
5.Skulachev, V. P. (1989) Energetics of Biological
Membranes [in Russian], Nauka, Moscow.
6.Skulachev, V. P. (1990) J. Membr. Biol.,
114, 97-112.
7.Bakeeva, L. E., and Brustovetsky, N. N. (1993)
Biol. Membr. (Moscow), 10, 36-43.
8.Markova, O. V., and Bakeeva, L. E. (1995) Biol.
Membr. (Moscow), 12, 138-146.
9.Crompton, M., McGuinners, O., and Nazareth, W.
(1992) Biochim. Biophys. Acta, 1101, 214-217.
10.Rodionov, I. N., Chentsov, Yu. S., Yarygin, V.
N., Bakeeva, L. E., Mukhammedov, A., Fedoseev, V. A., Lebedev, D. B.,
and Giber, L. M. (1982) Byull. Eksp. Biol. Med., No. 5,
34-37.
11.Paukov, V. S., and Gavrish, A. S. (1987) Abst.
IV All-Union Conf. Cell Pathology [in Russian], Nauka, Moscow, p.
66.
12.Arai, M., Gordon, E. R., and Lieber, C. S.
(1984) Biochim. Biophys. Acta, 797, 320-327.
13.Montgomery, R. I., Spach, P. I., and Cunningham,
C. C. (1984) Fed. Proc., 43, 1878.