Expression of Protooncogenes during Lymphocyte Activation by Growth Factors
E. G. Bulanova,1,2 V. M. Budagyan,1 A. A. Yarilin,1 and N. N. Mazurenko3
1Institute of Immunology, Russian Ministry of Health Care, Kashirskoe Shosse 24/2, Moscow, 115478 Russia; fax: (7-095) 117-1027.2To whom correspondence should be addressed.
3Institute of Carcinogenesis, Blokhin Oncology Research Center, Russian Academy of Medical Sciences, Kashirskoe Shosse 24, Moscow, 115478 Russia.
Submitted March 31, 1997; revision submitted June 16, 1997.
Effects of growth factors of non-immune origin including somatotropin (ST) and platelet-derived growth factor (PDGF) on the expression of the proteins encoded by c-fos, c-myc, c-fun, and c-ets family protooncogenes were studied for the first time. The dynamics of the oncoprotein expression in activated CD3+-lymphocytes was investigated by immunoblotting. The accumulation of the Fos and Myc proteins was enhanced in T-lymphocytes treated with ST, PDGF, or phytohemagglutinin; the accumulation was maximum at 30-60 min and decreased in 2 h; the data indicate that the oncoproteins participate in the early lymphocyte activation by various growth factors. The Jun protein appears only in 3 h after the onset of lymphocyte activation; this suggests independent participation of Fos in the early stages of lymphocyte activation prior to the appearance of Jun, preceding the joint action of Fos and Jun within the AP-1 transcription complex. The products of the c-ets family are differentially activated by the studied growth factors. Resting lymphocytes actively accumulate the Ets-1 protein; ST and PDGF activation decreases Ets-1 expression in 2 h. The Ets-2 protein is not detected in resting cells and PDGF-activated lymphocytes, whereas lymphocyte activation by ST is associated with accumulation of Ets-2. The data suggest that the product of the c-ets-1 gene is more important in the regulation of resting cells and the product of the c-ets-2 gene is important during activation of lymphocytes by ST. The results indicate that activation of lymphocytes with growth factors of non-immune origin is mediated by several signal transduction pathways.
KEY WORDS: lymphocyte activation, protooncogene, Myc, AP-1, Ets-1, Ets-2.
Abbreviations: ST) somatotropin; PDGF) platelet-derived growth factor; TPK) tyrosine protein kinases; PHA) phytohemagglutinin; IL-2) interleukin 2; DAB) diaminobenzidine; PBS) phosphate-buffered saline; PMSF) phenylmethylsulfonyl fluoride.
Interaction between an antigen and its appropriate receptor is the main
stimulus to activation and proliferation of the mature lymphocytes.
Antigen stimulation by itself can not start cell proliferation; it can
only bring resting lymphocytes into G1 phase of the cell
cycle (with the participation of coreceptors). During this change the
receptors for the growth factors are becoming expressed on their
surface, and only binding of the growth factors to these receptors
causes cell mitosis [1].
It is known that mature lymphocytes can multiply in the peripheral part of the immune system without following differentiation [2]. The nature of this phenomenon is not studied; it can be inferred that this proliferation is caused not by cytokines (participating in the immune response) but by growth factors (influencing cells of various histogenetic types). It would appear reasonable that this part can be played by the growth factors, for example, somatotropin (ST)4 or platelet-derived growth factor (PDGF); for these, surface lymphocyte receptors exist [3, 4].
Pathways for intracellular signal transduction during lymphocyte activation by antigens with the participation of cytokines (for example, interleukin 2 (IL-2)) and by non-immune growth factors are different, though they have common links [5]. Specifically, the spectrum of transcription factors responsible for the final stage of the signal transduction--expression of the genes participating in the cell activation and division--can be different because the set of genes starting this event may coincide only partially.
The production of the "early activation proteins", Fos, Myc, and Jun [6], and changes in the expression of the proteins encoded by the ets-1 and ets-2 genes [7] are registered during activation of lymphocytes with antigenic mitogens. The structure and features of proteins Ets-1 and Ets-2 are similar to the same of Fos and Myc proteins, though their function in lymphocyte activation is not clear. Activation of the Ras-pathway, with the participation of serine--threonine protein kinases cascade (MAP-kinases), is a necessary condition for the successful expression of the early genes. The degree of Ras-pathway participation in the conduction of a signal and function of the proteins encoded by the early genes in the process of the further activation can vary significantly just at the time of the action of interleukins on the activated lymphocytes. Thus, these protein products participate in signal transduction from interleukin 2, but not from interleukin 4 [8]. The degree of involvement of the mentioned genes and their products in activation of lymphocytes by other growth factors is not clear, especially when it is considered that there are differences in the structure of the receptors for ST and PDGF (the first one belongs to the family of cytokine receptors and appears to be lacking in its own enzymatic activity, whereas the cytoplasmic domain of the second demonstrates activity of the tyrosine protein kinase) [4, 9]. The goal of this work was to evaluate expression of the early activation proteins, Myc, Fos, and Jun, as well as Ets-1 and Ets-2 under activation of T-lymphocytes from the human blood with growth factors of non-immune origin, ST and PDGF.
MATERIALS AND METHODS
Mononuclear cells of peripheral blood were isolated from venous heparinized blood of healthy donors using density-gradient centrifugation at 400g and 20°C for 30 min in Ficoll (Pharmacia, Sweden) and Verographine (Spofa, Czechia) (d = 1.077). Cell suspension from the interphase was washed three times for 10 min with medium 199 at 200g. Monocytes were removed by adhesion on plastic at 37°C for 40 min. B-Lymphocytes and initially activated T-lymphocytes were removed according to the method of negative selection [10] by successively treating the suspension with anti-CD22 and anti-CD25 mouse monoclonal antibodies (NPTs MedBioSpektr, Russia) with subsequent adhesion on plastic covered with rabbit antibodies against mouse Ig. The non-adhered cells were gathered, resuspended in the cultural medium containing RPMI 1640 medium with 2 mM L-glutamine, 20 mM HEPES (all from Flow Labs, Scotland), and gentamicin (80 µg/ml) from Pharmachim (Bulgaria). The resulting cell population consisted of CD3+-T-lymphocytes to the extent of 95%; the number of viable cells not stained with trypan blue was more than 98%.
The cells were pre-incubated for 2 h in the poor medium 199 in the presence of 100 µM of sodium ortho-vanadate from Aldrich (USA), with following twofold washing. One-milliliter aliquots of the cell suspension (5·106 cells/ml) were applied into the wells of the 24-well plate for cell cultures (Linbro, Scotland) with 10 µg of ST (kindly provided by A. V. Balura, Institute of Immunology, Moscow), or with 5 µg of PDGF from Boehringer Mannheim (Germany), or with 20 µg of phytohemagglutinin (PHA) from Sigma (USA). Then the plate was incubated in a CO2-incubator for different periods of time and washed twice with medium 199. Then the cell precipitate was lysed with RIPA buffer (0.15 M NaCl, 0.05 M Tris, pH 7.4, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 1% deoxycholate, and 1 mM phenylmethylsulfonyl fluoride (PMSF), all from Sigma) containing 100 µM of sodium ortho-vanadate. The lysates were stored at -70°C. Buffer including 11% glycerol, 2% SDS, 0.2% bromphenol blue, 5% beta-mercaptoethanol, and 0.05 M Tris-HCl, pH 7.4, was added before PAGE and samples were heated to 100°C for 5 min.
Oncoproteins were detected by immunoblotting [11]. The proteins were separated by SDS-PAGE (10% gel) according to Laemmli (100 µg of protein per lane) for 3 h at 4°C at 100 mA and 50 V. Proteins from the gel were transferred onto a nitrocellulose membrane from Schleicher and Schuell (Germany) in 0.04 M phosphate buffer, pH 6.5, for 4 h at 700 mA using a Transfor instrument (LKB, Sweden). To prove the completeness of the protein transfer from the gel and to evaluate protein content in the lanes, blots were stained by Ponceau and washed with distilled water. Phosphate-buffered saline (PBS) containing 0.05% Tween-20 was used for blocking, dilution of antibodies, and washing of blots. To prevent nonspecific binding of antibodies, blots were blocked for no less than 2 h with 5% fat-free milk from Amersham (England) and then incubated with polyclonal rabbit antisera against fragments of oncoproteins Fos (amino acids 6-14), Myc (amino acids 57-68), Jun (amino acids 1-14), Ets-1 (amino acids 432-441), and Ets-2 (amino acids 460-469) obtained in the Laboratory of Molecular Biology of Viruses, Institute of Carcinogenesis, Oncology Research Center [11-13]. Antibodies against oncoproteins were diluted 1:200.
Goat anti-rabbit IgG conjugated to horseradish peroxidase from Sigma (dilution 1:3000) was used as the second antibodies. Then blots were stained with buffer containing diaminobenzidine (DAB) (1 mg of DAB per 10 ml of PBS with 10 µl of 33% H2O2).
The results of the study were processed using the MD QuantImage program, version 3.3 (Molecular Dynamics, USA) and are represented as plots. The amount of protein per lane were compensated for intensity of staining of the background and the areas corresponding to the studied proteins were considered.
RESULTS
We have evaluated expression of protooncogenes of the transcription factors c-fos, c-myc, c-jun, c-ets-1, and c-ets-2 during activation of lymphocytes with ST and PDGF using specific polyclonal antibodies against oncoproteins. For comparison, activation of lymphocytes with PHA (mitogenic lectin) which is capable of binding to the sugar residues of the surface receptors including T-cell receptor and thus to cause cell activation [14] was studied in some experiments.
In previous experiments it was found that the total pool of mononuclear leukocytes contains a high level of oncoproteins: Fos and Myc in activated lymphocytes (CD25+ cells, ~5%) and monocytes (~12%). Thus we removed these cells by negative selection using specific antibodies and adhesion on plastic. The cell population obtained by these means contained ~95% CD3+-lymphocytes. Addition of calf embryonal serum into the culture medium also affects the rate of oncoprotein synthesis (the serum includes growth factors such as insulin-like growth factors and epidermal growth factor favoring activation of lymphocytes). In this connection activation of the cell cultures was preceded by pre-incubation in the poor serum-free medium. To prevent dephosphorylation of the proteins with phosphatases 100 µM of sodium ortho-vanadate was added to the pre-incubation medium.
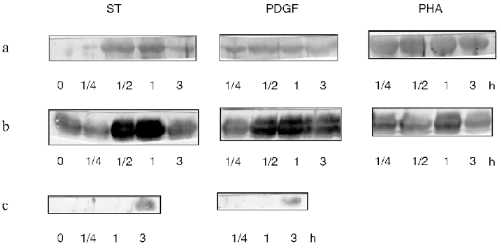
Figure 1 demonstrates results of immunoblotting the protein using specific antibodies against fragments of Fos, Myc, and Jun which revealed p62c-fos, p64c-myc, and p45c-jun proteins. Resting lymphocytes prior to adding studied growth factors served as a control. The intensity of the staining was proportional to the amount of added protein. As seen from these data, initially CD3+-lymphocytes contain low level of Fos protein, whereas Myc and Jun proteins are not detectable in resting cells. Increasing of Fos with a maximum at 1 h and of Myc at 30 min is noted under the influence of ST. The content of the both proteins is decreasing within 2 h. In the case of Jun protein the situation is different: it appears only in 2 h of lymphocyte activation with ST. During lymphocyte activation with PDGF increase in Fos and Myc oncoproteins content with a maximum at 30 min is noted. As in the case of ST activation, the amount of the proteins is decreasing in 2 h. In this case Jun protein also appears after 2 h of activation. During activation of lymphocytes with PHA, both proteins, Fos and Myc, appear in just 15 min after addition of the factor and their amount is decreasing at 2 h.
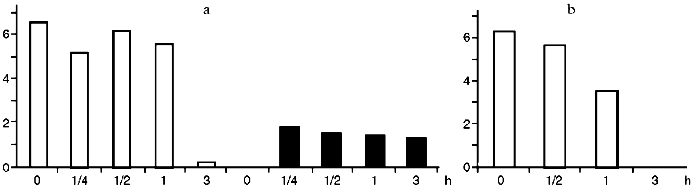
To evaluate the dynamics of Ets-1 and Ets-2 oncoproteins synthesis we scanned the blots and processed our data using the program MD QuantImage. Resting lymphocytes contain Ets-1 (p55) at a high level (Fig. 2). On the contrary, Ets-2 (p54-56) is practically undetectable in resting cells as well as after its activation with PDGF. During activation of lymphocytes with PDGF the content of Ets-1 decreased drastically in 2 h. During activation of lymphocytes with ST, decreasing of Ets-1 and increasing of Ets-2 were noted in 2 h, beginning 15 min after the onset of the cell activation.Fig. 1. Results of immunoblotting of oncoproteins Myc (a), Fos (b), and Jun (c) from CD3+-lymphocytes lysed after different intervals after their activation with ST, PDGF, and PHA. Time (in hours) is indicated under each blot. The zero point corresponds to resting lymphocytes.
Fig. 2. Change in the content of oncoproteins Ets-1 (unfilled bars) and Ets-2 (filler bars) during activation of lymphocytes with ST (a) and PDGF (b). The results of immunoblotting were scanned and normalized considering protein concentration in each lane after staining with Ponceau dye. Abscissa, time of lymphocyte activation with growth factors (h); ordinate, the conventional units obtained from data processing using MD QuantImage, which intergrates the intensity of the protein area staining.
DISCUSSION
Recently much attention has been given to the study of pathways for signal transduction from receptors of growth factors, because it is precisely these endogenic growth factors that can mediate cell activation in response to antigen. As mentioned above, only the interaction of IL-2 (which is the main growth factor of T-lymphocytes) with its receptor causes transfer of T-lymphocytes into the S-phase of the cell cycle and mitosis advantageous [15]. It was shown that the action of the transcription factors encoded by the "early response genes" on the enhancer regions of the gene coding for IL-2 is necessary for the induction of the transcription of this gene. The most important transcription factors are Fos, Jun, and Myc [16]. We have studied the influence of endogenic growth factors (ST and PDGF) on the change in the content of oncoproteins--transcription factors Fos, Jun, and Myc--and Ets protein family (Ets-1 and Ets-2) in the lymphocytes during their activation. The use of the known pathways for signal transduction during activation of T-lymphocytes with these factors is yet to be explained.
The results of the research on the kinetics of protooncogene expression in lymphocytes are discrepant. Thus, previously rapid increasing of c-fos expression with a peak at 15 min (1 h after the onset of lymphocyte activation [17-19]) and its decreasing to the basal level in 2 h [19] was noted. At the same time, other authors mentioned a later increase of the Fos and Myc proteins with a peak in 6-9 h [20]. Expression of c-jun was registered in just 30 min from the onset of activation and increased in 6 h simultaneously with decreasing of c-fos to the basal level [21]. These differences are likely to be connected with variations in the experimental models and the set of the growth factors used. We originally studied changes in the content of oncoproteins during activation of T-lymphocytes with ST and PDGF--growth factors of distinct origin and action. The system which we have used (isolation of CD3+-lymphocytes population, pre-incubation of the cells in a poor medium) allowed removal of various intrinsic and extrinsic factors affecting the activation process and enabled us to study the action of the growth factors chosen by us on a cell. Previously it was more common to use the whole pool of mononuclear cells which contain not only lymphocytes; mitogens (PHA) were used as activation stimulus or to simulate the action of antigen (the cell was activated with anti-CD3 antibodies) [18-21]. Furthermore, most of the authors judged the expression of protooncogenes from the presence of mRNA in the cell. We have determined the end product--protein--of activation of the corresponding protooncogene.
Of interest is the fact that Fos protein is noted in abundance 15 min after ST and PDGF addition, with its maximum in 1 h and decrease at 2 h, whereas appearance of Jun protein is noted only in 3 h after the beginning of activation. Both of these proteins usually act as a part of the transcription factor AP-1, which can include either heterodimer Fos--Jun, or homodimer Jun--Jun, but not Fos--Fos [6, 22]. Thus, it can be proposed that in this case Fos acts as a transcription factor in its own right at the early stages. In the literature there is evidence that Fos can bind with TATA-regions of promoters of various genes [23]. Nevertheless, it is possible that AP-1 containing Jun--Jun homodimer or Fos-Jun heterodimer appears in the cell after the production of Jun, and it is precisely this moment that starts the transfer of lymphocytes into the G1-phase of the cell cycle ending with expression of the IL-2 gene. The related temporal changes in the expression of Fos and Jun proteins were found previously [21].
We detected similarity in the kinetics of Fos and Myc protein expression during activation of lymphocytes with growth factors ST and PDGF. In studies of fibroblasts activated with PDGF it was shown that expression of c-fos and c-myc genes results from activation of different signal pathways (Ras- and Src-dependent, respectively) [24]. On this basis it is believed that, in spite of differences in the receptor structures (ST receptor is in the family of cytokine receptors [9] while PDGF receptor is a TPK receptor [4]), intracellular activation routes resulting in expression of c-fos, c-jun, and c-myc are quite similar and involve Ras- and Src-kinases into activation. It is known that activation of only one pathway (Ras- or Scr-dependent) does not result in efficient DNA synthesis and subsequent mitosis [24].
The function of protooncogenes from the c-ets family in the activation of lymphocytes by mitogens and growth factors seems to be more conjectural. Products of c-ets-1 and c-ets-2 genes are factors of transcription, resembling Fos and Jun proteins in properties and coming into play during maturation and differentiation of the lymphocyte precursors in the thymus [7, 25]. The data about expression of these genes in mature peripheral lymphocytes are discrepant. According to our results, Ets-1 protein can be found in abundance in resting lymphocytes; in contrast, Ets-2 protein is not found; this supports other data [7, 26]. Decreasing of Ets-1 to its nearly full disappearance in 2 h was noted during activation of lymphocytes with ST and PDGF, which is in agreement with previously obtained data [7] about decreasing expression of this particular protooncogene during antigen stimulation of lymphocytes through the T-cell receptor. At the same time, it was shown [27], that ets-1 (but not ets-2) gene is induced during antigen stimulation with a T-cell hybrid. In spite of these differences, it is suggested that the c-ets-1 gene is defined as one of the regulators for the G0 phase of the cell cycle.
Activation of lymphocytes leads to the rapid accumulation of Ets-2 protein in just 15 min after addition of the factor. During activation of lymphocytes with PDGF the protein is not found. Thus, the c-ets-2 gene is important for activation of lymphocytes with ST rather than PDGF.
The experimental data given in this paper indicate that there are several pathways for lymphocyte activation with growth factors of non-immune origin. These pathways are interrelated and complement each other; their combined action defines expression of the transcription factors called "early activating genes" (c-fos, c-jun, c-myc, c-ets-1, and c-ets-2). The function of the latter is to provide advantageous transfer of the cell into the S-phase of the cell cycle and its consequent proliferation.
This work was supported by the Russian Foundation for Basic Research (grants 96-04-49163 and 95-04-13085a). The authors express gratitude to Dr. F. L. Kiselev (Institute of Carcinogenesis, Blokhin Oncology Research Center, Russian Academy of Medical Sciences, Moscow) for comprehensive assistance in this work. We thank E. V. Musatkina for technical assistance in processing of the data.
LITERATURE CITED
1. Robey, E., and Alison, J. P. (1995) Immunol.
Today, 16, 306-313.
2. Rocha, B., Dautigni, N., and Pereira, P. (1989)
Eur. J. Immunol., 19, 905-911.
3. Mercola, K. E., Cline, M. J., and Golde, D. W.
(1981) Blood, 58, 337-340.
4. Habernicht, A. J. R., Salbach, P., Blattner, C.,
and Janben-Tummer, U. (1989) in Growth Factors, Differentiation
Factors and Cytokines (Habernicht, A. J. R., ed.) Springer-Verlag,
Berlin, pp. 31-41.
5. Ihle, J. N., Withuhn, B. A., Quell, F. W.,
Yamamoto, U., and Silvennoinen, O. (1995) Ann. Rev. Immunol.,
13, 369-398.
6. Karin, M., and Smeal, T. (1992) Trends Biochem.
Sci., 17, 418-422.
7. Bhat, N. K., Thompson, G. B., Lindsten, T., June,
C. H., Fujiwara, S., Koizumi, S., Fisher, R. J., and Papas, T. S.
(1990) Proc. Natl. Acad. Sci. USA, 87, 3723-3727.
8. Satoh, T., Nakafuku, M., Migajima, A., and Kaziro,
I. (1991) Proc. Natl. Acad. Sci. USA, 88, 3314-3318.
9. Basan, J. F. (1989) Biochem. Biophys. Res.
Commun., 164, 788-795.
10. Maison, D., Penheil, J., and Seduik, J. (1990)
Lymphocytes: Methods (Klaus, J., ed.) [Russian translation],
Mir, Moscow, pp. 69-96.
11. Mazurenko, N. N., Kiseleva, N. P., Kudryavets,
Yu. I., Mikhaleva, I. I., Antonenko, V. V., and Kiselev, F. L. (1990)
Mol. Biol. (Moscow), 24, 1351-1362.
12. Mazurenko, N. N., Kogan, E. A., Sukhova, N. M.,
and Zborovskaya, I. B. (1992) Vopr. Med. Khim., 1,
53-59.
13. Mazurenko, N. N., Fedorov, S. P., Bogoslovskii,
B. P., Knyazev, P. G., and Seitts, I. F. (1986) Eksp. Onkol.,
8, 18-21.
14. Ling, N. R. (1973) Lymphocyte
Stimulation, North-Holland Publ., Amsterdam.
15.Altman, A., Mustelin, T., and Coggeshall, K. M.
(1990) Crit. Rev. Immunol., 10, 347-391.
16. Mills, G. B., Schmandt, R., Gibson, S., Leung,
B., Hill, M., May, C., Shi, Y. F., Branch, D. R., Radvanyi, L., Truitt,
K. E., and Imboden, J. (1993) Semin. Immunol., 5,
345-364.
17. Reed, J. C., Alpers, J. D., Nowell, P. C., and
Hoover, R. G. (1986) Proc. Natl. Acad. Sci. USA, 83,
3982-3988.
18. Kovary, K., and Bravo, R. (1991) Mol. Cell.
Biol., 11, 2451-2459.
19. Prywes, R., Fisch, T. M., and Roeder, R. G.
(1988) in Molecular Biology of Signal Transduction, Vol. 54,
Cold Spring Harbor, New York, pp. 739-743.
20. Lynch, D. C., Wallace, D. L., and O'Flynn, K.
(1988) in Molecular Biology of Signal Transduction, Vol. 54,
Cold Spring Harbor, New York, pp. 137-159.
21. Song, L., Stefens, J. M., Kittur, S., Collins,
G. D., and Nagel, J. E. (1992) Mech. Ageing Dev., 65,
149-156.
22. Auwerx, J., and Sassone-Corsi, P. (1992)
Oncogene, 7, 2271-2280.
23. Metz, R., Kouzarides, T., and Bravo, R. (1994)
EMBO J., 13, 3832-3842.
24. Barone, M. V., and Courtneidge, S. A. (1995)
Nature, 378, 509-512.
25. Bhat, N. K., Komschlies, K. L., Fujiwara, S.,
Fisher, R. J., Mathienson, B. J., Gregorio, T. A., Young, H. A., Kasik,
J. W., Ozato, K., and Papas, T. S. (1989) J. Immunol.,
142, 672-678.
26. Sacchi, S., de Klein, A., Showalter, S. D.,
Bigi, G., and Papas, T. S. (1988) Leukemia, 2,
12-16.
27. Kaufmann, Y., Silverman, T., Levi, B., and
Ozato, K. (1987) J. Exp. Med., 166, 810-814.