Covalent Modification of Superoxide Dismutase Subunits by Chondroitin Sulfate
A. V. Maksimenko1,2 and E. G. Tischenko1
1Institute of Experimental Cardiology, Russian Cardiology Research Center, ul. 3-ya Cherepkovskaya 15a, Moscow, 121552 Russia; fax: (095) 415-2962; E-mail: csc@adonis.ias.msk.su2To whom correspondence should be addressed.
Submitted July 2, 1997; revision submitted July 16, 1997.
The interaction of superoxide dismutase with sodium chondroitin sulfate was studied. The enzyme easily forms both enzyme associations and non-covalent complexes with chondroitin sulfate in solution. The enzyme was chemically modified with benzoquinone-activated chondroitin sulfate. The electrophoresis and ultrafiltration data indicate the formation of covalently modified derivatives of superoxide dismutase. Almost half of the superoxide dismutase subunits were covalently bound to chondroitin sulfate; the modified subunit retained the ability to form dimers with the native subunit. The modified superoxide dismutase possesses high residual catalytic activity and is promising for biomedical investigations.
KEY WORDS: superoxide dismutase, chondroitin sulfate, glycosaminoglycans, chemical modification, covalent conjugate.
The antioxidant enzyme superoxide dismutase (SOD) catalyzes disproportionation of superoxide radical [1, 2], thus protecting cells, tissues, and biomacromolecules from free radical damage [3, 4]. One of the common sites where such damage develops is the endothelial surface [5-7]. SOD binds to a cell surface via interaction with glycosaminoglycans (GAG) [8, 9]. GAG are the carriers of the negative charge of the vessel wall [10], possess athrombogenic [11, 12], anti-thrombotic [13-16], and anti-atherosclerotic effects [17-21], and are used in clinical practice [13, 22]. Hence, GAG are promising agents for modification of SOD to enhance the antioxidant protection of the endothelial surface of the vessel wall. In fact, the outcome of experimental therapy with SOD is enhanced after its modification [23] due to the accumulation of the substance in the damaged sites [24-26], increase in its retention in the body [27, 28], and increase in enzyme stability [29, 30]. These biological effects of SOD and GAG can enhance the therapeutic activity of the compounds during combined administration and increase the antithrombotic protection of the vessel wall. Thus, investigation of the interaction of SOD with GAG is of interest for biochemistry and pharmacology. Chondroitin sulfate (CS) was selected as a representative of the GAG family because it is accumulated preferentially in the vessel wall [31] and not so abundant versus dermatan sulfate in atherosclerotic plaques [32]; its interaction with the enzymes was studied previously in our laboratory [33]. It was shown that this GAG can covalently modify acid phosphatase [34].
Molecular construction of medical formulations by covalent cross-linking of various physiologically active compounds demonstrated promise of this approach for the development of new formulations with combined activity [35]. This results in manifestation of therapeutic activities of all formulation components including the enzyme [28] and the modifier [36]. Similar modification was successful in the case of SOD [23]. After covalent binding of SOD to Ficoll [37], the latter inhibited neutrophil adhesion to endothelium whereas SOD suppressed the superoxide radical accumulation in sheep pulmonary microembolism. Combined administration of heparin and thrombolytic agents inhibited superoxide radical formation during neutrophil activation [38]. This effect of GAG can enhance and supplement the action of the antioxidant enzymes. Thus, use of SOD and CS for the development of new antithrombotic formulations is reasonable and interesting.
The formation of the complex between SOD and CS and preparation of SOD treated with CS was the goal of the present work.
MATERIALS AND METHODS
Cu,Zn-superoxide dismutase was isolated from rat liver (molecular weight 32 kD) [39]; sodium chondroitin sulfate (chonsuride, chondroitin sulfate A, molecular weight 30 kD) was from St. Petersburg Meat Processing Plant; benzoquinone, dimethylformamide, and xanthine were from Sigma (USA); xanthine oxidase was from Calbiochem (USA); nitrotetrazolium blue was from Reanal (Hungary); Sephadexes G-25 and G-100 were from Pharmacia (Sweden). All other reagents were of analytical grade from Reakhim (Russia).
Protein content was assayed by the Bradford method [40]. SOD activity was assayed by inhibition of nitrotetrazolium blue reduction during generation of superoxide radical in the xanthine/xanthine oxidase system at pH 7.8 as described earlier [30, 33]. One unit of SOD activity corresponds to the amount of the enzyme required for 50% suppression of nitrotetrazolium blue reduction.
SOD was treated with chondroitin sulfate as follows. First, 100 mg of benzoquinone dissolved in 3 ml of dimethylformamide were added to 100 mg of chondroitin sulfate dissolved in 6 ml of 0.02 M phosphate buffer (pH 6.0); the mixture was incubated for 1.5 h at room temperature in the dark. Second, activated CS was isolated on a Sephadex G-25 column equilibrated with the same buffer. Third, SOD was reacted with activated CS. SOD (7.5 mg protein) was added to 10 ml of purified activated CS and the pH of the mixture was adjusted to 9.5 with 1 M sodium carbonate; the mixture was incubated for 24 h at room temperature in the dark. SOD concentration in the incubation medium was 22.5 µM and CS concentration was 0.33 mM. The product was purified by gel filtration on a Sephadex G-100 column (eluted with 0.05 M phosphate buffer, pH 7.5) and lyophilized (SOD--CS conjugate).
Large scale production of the SOD complex with benzoquinone-activated CS was done using an ultrafiltration apparatus (Amicon, USA) with an XM-50 membrane. The membrane was washed with 0.025 M sodium phosphate and 0.075 M NaCl (pH 7.5) until absorption at 280 nm was less than 5% of the initial value and the supernatant was lyophilized.
Comparative ultrafiltrational separation of native SOD, a mixture of SOD and CS, and SOD--CS conjugate was performed using an XM-30 membrane. The washing solution contained 0.025 M sodium phosphate and 0.075 M NaCl (pH 7.5) with or without 3 M NaCl. When the optical density at 280 nm of the wash-through became constant, the separation was stopped; the protein content in the solutions above and below the membrane was assayed by the Bradford method [40].
Electrophoretic separation of the adducts was performed using 5-20% polyacrylamide gel with (denaturing electrophoresis) or without (native electrophoresis) SDS [41]; gels were scanned in a 2202 Ultrascan laser densitometer (LKB, Sweden).
RESULTS AND DISCUSSION
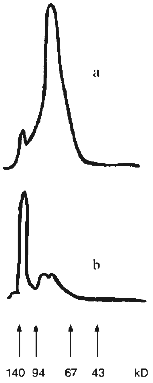
Carbodiimide cannot be used to covalently conjugate SOD and CS [33]. Hence, we coupled SOD and CS by benzoquinone activation of GAG; this should have resulted in an essentially homogenous adduct and preservation of a significant portion of residual catalytic activity [42]. Cu,Zn-SOD from rat liver is an oxidoreductase composed of two identical 16 kD subunits. Catalytic activity is attributable to the dimeric SOD molecule but not to a single subunit. According to native electrophoresis, SOD easily self associates. This is supported by the densitogram of native electrophoresis (Fig. 1a). Almost all protein (over 95%) is located at the band of the associates of four-six SOD subunits. Comparison with the densitogram of native electrophoresis of the SOD--CS conjugate (Fig. 1b) clearly indicates the presence of the high molecular weight fraction (at least 60% of the total area of the adduct peaks). However, it is difficult to conclude that the interaction of SOD with CS is covalent because this reagent easily forms non-covalent complexes detected by gel filtration [33].
Electrostatic interactions initiate the formation of such a complex. The impact of these interactions on the complex formation between SOD and CS was evaluated. The preparations of native SOD, mixture of SOD and CS (SOD + CS), and SOD treated with benzoquinone-activated CS (SOD--CS conjugate) were subjected to comparative ultrafiltrational separation using an XM-30 membrane (molecular weight of the dimeric molecule of native SOD is 32 kD). The data of Table 1 indicate that electrostatic interactions play a significant role in interaction of the SOD subunits with each other. Increasing the ionic strength of the solution significantly weakens the interactions. However, residual complex formation was detected under these conditions in the mixture of SOD and CS and to a higher extent for the SOD--CS conjugate. Comparison of the separation of the mixture of SOD and CS and SOD--CS conjugate indicates that the formation of the complex of these reagents is complicated and cannot be due to a single type of interaction even in a simple mixture of the reagents.Fig. 1. Densitograms of native electrophoresis of native SOD (a) and SOD treated with activated CS (b). Arrows correspond to the positions of marker proteins.
TABLE 1. Ultrafiltrational Separation of
SOD and CS Preparations by an Amicon Apparatus Using an XM-30
Membrane

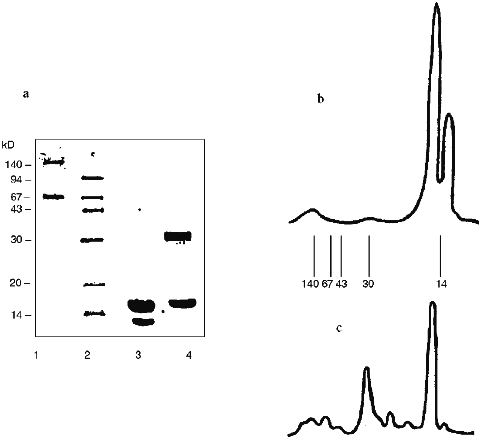
The data of denaturing electrophoresis (Fig. 2) indicate the presence of the covalently modified forms of SOD. Almost all native SOD dissociates into subunits (Figs. 2a (lane 3) and 2b). Treatment of SOD with activated CS results in the covalent modification of the enzyme (Figs. 2a (lane 4) and 2c). Covalently modified protein corresponds to 41% of the total peak area; this is in good agreement with the ultrafiltration data (Table 1). Evidently, cross-linked CS dimers (or other oligomers), SOD (minor part, about 10%, Fig. 2c), modified CS, and native subunits of the enzyme (major part, over 30%, Fig. 2c) are present in the SOD--CS conjugate sample. Modified subunits can efficiently interact with the native subunits; their sum would correspond to the major part of the preparation (Fig. 2, a and c). Self association of SOD in the solution (Fig. 1a) can prevent the cross-linking of the dimer molecule by CS. Under these conditions, the enzyme subunits are predominantly modified by CS (Fig. 2, a and c).
Thus, benzoquinone coupling of SOD and CS results in a modified enzyme form whose residual catalytic activity is 80-82% of the initial. The data emphasize the efficiency of interaction of the modified and native SOD subunits with each other; because of this, the SOD--CS conjugate has high residual activity. Specific activity of native SOD is 1600 U/mg protein and that of the SOD--CS conjugate is 1300 U/mg protein (protein content in the preparation is 5%). Total product yield was about 80%. The preparation of SOD conjugated with CS is promising for biomedical investigations; the method of covalent modification of the enzyme allows preparation of significant amounts of the SOD--CS conjugate for comparative evaluation of its activity in vivo. This is the next stage of the study.Fig. 2. Electrophoregram of denaturing electrophoresis of SOD preparations (a) and densitograms of native SOD (b) and SOD--CS conjugate (c). a: 1, 2) Protein markers; 3) native SOD; 4) SOD--CS conjugate; b, c) lines correspond to positions of the marker proteins.
This work was supported in part by the State Scientific and Technological Program "National Priorities in Medicine and Health Care", section "Atherosclerosis" (grants No. 009 and 508) and by the Ministry of Health Care and Medical Industry of the Russian Federation.
LITERATURE CITED
1.Fridovich, I. (1986) Arch. Biochem.
Biophys., 247, 1-11.
2.Halliwell, B., and Gutteridge, J. M. C. (1986)
Arch. Biochem. Biophys., 246, 501-514.
3.Opie, L. (1989) Circulation, 80,
1049-1062.
4.Kloner, R. A., Przyklenk, K., and Whittaker, P.
(1989) Circulation, 80, 1115-1127.
5.Yu, B. P. (1994) Physiol. Rev., 74,
139-162.
6.Sies, H. (1993) Eur. J. Biochem.,
215, 213-219.
7.Boissinot, M., Kuhn, L. A., Lee, P., Fisher, C. L.,
Wang, Y., Hallewell, R. A., and Tainer J. A. (1993) Biochem.
Biophys. Res. Commun., 190, 250-256.
8.Inoue, M., Watanabe, N., Matsuno, K., Sasaki, J.,
Tanaka, Y., Hatanaka, H., and Amachi, T. (1991) J. Biol. Chem.,
266, 16409-16414.
9.Karlsson, K., Sandstrom, J., Edlund, A., and
Marklund, S. L. (1994) Lab. Invest., 70, 705-710.
10.Danon, D., and Skutelsky, E. (1976) Ann. N. Y.
Acad. Sci., 275, 47-63.
11.Heyderman, R. S., Klein, N. Y., and Levin, M.
(1992) Thrombos. Res., 87, 677-685.
12.Bourin, M.-C., and Lindahl, U. (1993) Biochem.
J., 289, 313-330.
13.Bianchini, P. (1989) Semin. Thrombos.
Hemostas., 15, 365-369.
14.Gebbink, R. K., Reynolds, C. H., Tollefsen, D.
M., Mertens, K., and Pannekoek, H. (1993) Biochemistry,
32, 1675-1680.
15.Fernandes, F., N'guyen, P., Van Ryn J., Ofosu, F.
A., Hirsh, J., and Buchanan, M. R. (1986) Thrombos. Res.,
43, 491-495.
16.Edelberg, J. M., Weissler, M., and Pizzo, S. V.
(1991) Biochem. J., 276, 785-791.
17.Matsushima, T., Nakashima, Y., Sugano, M.,
Tasaki, H., Kuroiwa, A., and Koide, O. (1987) Artery,
14, 316-337.
18.Ross, R. (1993) Nature, 362,
801-809.
19.Morrison, L. M. (1971) Angiology,
22, 165-174.
20.Day, C. E., Powell, J. R., and Levy, R. S. (1975)
Artery, 1, 126-137.
21.Morrison, L. M., and Schjeide, O. A. (1974)
Coronary Heart Disease and the Mucopolysaccharides
(Glycosaminoglycans), Charles C. Thomas, Springfield, II.
22.Ivankin, A. N., Vasyukov, S. E., and Panov, V. P.
(1985) Khim.-Farm. Zh., 19, 192-202.
23.Maksimenko, A. V. (1993) Usp. Sovrem.
Biol., 113, 351-365.
24.Fujita, T., Furutsu, H., Nishikawa, M., Takakura,
Y., Sezaki, H., and Hashida, M. (1992) Biochem. Biophys. Res.
Commun., 189, 191-196.
25.Igarashi, R., Hoshino, J., Takenaga, M., Kawai,
S., Morizawa, Y., Yasuda, A., Otani, M., and Mizushima, Y. (1992) J.
Pharmacol. Exp. Ther., 262, 1214-1219.
26.Maksimenko, A. V., Petrov, A. D., Caliceti, P.,
Konovalova, G. G., Grigorieva, E. L., Schiavon, O., Tischenko, E. G.,
Lankin, V. Z., and Veronese, F. M. (1995) Drug Delivery,
2, 39-43.
27.Fujita, T., Nishikawa, M., Tamaki, C., Takakura,
Y., Hashida, M., and Sezaki, H. (1992) J. Pharmacol. Exp. Ther.,
263, 971-978.
28.Maksimenko, A. V., Bezrukavnikova, L. M.,
Grigorieva, E. L., Yaglov, V. V., and Torchilin, V. P. (1992) Ann.
N. Y. Acad. Sci., 672, 118-125.
29.Veronese, F. M., Caliceti, P., Pastirino, A.,
Schiavon, O., Sartore, L., Banci, L., and Scolaro, L. M. (1989) J.
Contr. Rel., 10, 145-154.
30. Maksimenko, A. V., Grigorieva, E. L., Morozkin,
A. D., Tischenko, E. G., Minkovsky, E. B., and Torchilin, V. P. (1991)
Biokhimiya, 56, 1330-1336.
31.Lark, M. W., Yeo, T.-K., Mar, H., Lara, S.,
Hellstrom, I., Hellstrom, K.-E., and Wight, T. N. (1988) J.
Histochem. Cytochem., 36, 1211-1221.
32.Badimon, J. J., Fuster, V., Chesebro, J. H., and
Badimon, L. (1993) Circulation, 87 (Suppl. II),
II-3-II-16.
33. Maksimenko, A. V., Terentyeva, E. L.,
Konovalova, O. Yu., and Torchilin, V. P. (1988) Ukr. Biokh. Zh.,
60, 20-25.
34.Luchter-Wasylewska, E., Dulinska, J., Ostrowski,
W. S., Torchilin, V. P., and Trubetskoy, V. S. (1991) Biotechnol.
Appl. Biochem., 13, 36-47.
35.Maksimenko, A. V. (1994) Khim.-Farm. Zh.,
28, 3-13.
36.Maksimenko, A. V., and Torchilin, V. P. (1985)
Thrombos. Res., 38, 277-288.
37.Johnson, A., Perlman, M. B., Blumenstock, F. A.,
and Malik, A. B. (1986) Circ. Res., 59, 405-415.
38.Riesenberg, K., Schlaeffer, F., Katz, A., and
Levy, R. (1995) Br. Heart J., 73, 14-19.
39.Simonyan, M. A. (1984) Biokhimiya,
49, 1792-1798.
40.Bradford, M. M. (1976) Anal. Biochem.,
72, 248-254.
41.Laemmli, U. K. (1970) Nature, 227,
680-685.
42.Ternynck, T., and Avrameas, S. (1977)
Immunochem., 14, 767-773.