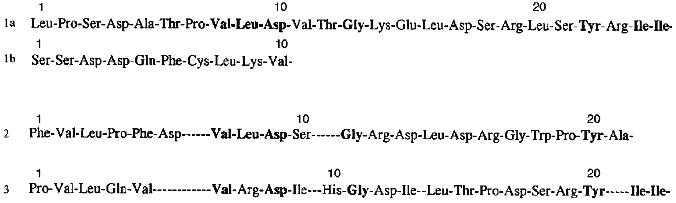Potato Tuber Protein Proteinase Inhibitors Belonging to the Kunitz Soybean Inhibitor Family
T. A. Valueva,1,2 T. A. Revina,1 and V. V. Mosolov1
1Bakh Institute of Biochemistry, Russian Academy of Sciences, Leninskii pr. 33, Moscow, 117071 Russia; fax: (095) 954-2732; E-mail: inbio@glas.apc.org2To whom correspondence should be addressed.
Submitted July 1, 1997.
Three protein inhibitors of proteolytic enzymes with molecular weights 21, 22, and 23 kD were isolated from potato tubers (Solanum tuberosum L.) by ammonium sulfate precipitation followed by gel and ion-exchange chromatography. The 21- and 22-kD proteins were shown to be serine proteinase inhibitors with different specificities. The 21-kD protein inhibits human leucocyte elastase and trypsin effectively, but it is less effective towards chymotrypsin. The 22-kD protein is an inhibitor of cysteine proteinases and suppresses the activities of papain, ficin, and bromelain with the same affinities. None of the isolated proteins inhibit subtilisin, pepsin, or cathepsin D. The 21-kD protein consists of two disulfide-linked polypeptide chains with molecular weights of 16.5 ± 1 kD and 4.5 ± 1 kD. The 22-kD and 23-kD proteins have a single polypeptide chain. The N-terminal 22-25 amino acid sequences of these three proteins were determined. These sequences have significant homology to other plant inhibitors from the Kunitz soybean inhibitor superfamily.
KEY WORDS: proteinase inhibitors, serine proteinases, cysteine proteinases, potato tuber.
Inhibitors of proteolytic enzymes have been found in plants from different families. The group of plant proteins have different properties. All plant protein proteinase inhibitors can be divided into at least ten structural groups (families) on the basis of their primary structure, number of disulfide bonds and their localization, and the disposition of their reaction centers [1]. Protein inhibitors of proteinases from the Kunitz soybean inhibitor superfamily (STI) form one of these groups [2]. Inhibitors from this superfamily with molecular weights 20-24 kD and two disulfide bonds were previously found mainly in legumes and cereals [2-5]. Currently several proteins from potato tubers with molecular weights from 20 to 24 kD were classified as serine (trypsin, chymotrypsin [6, 7], and subtilisin [6]), aspartyl (cathepsin D [8, 9]), and cysteine (papain and cathepsin L [10]) proteinase inhibitors. All these protein inhibitors are believed to be members of the Kunitz proteinase inhibitor superfamily on the basis of some features of their primary structure [6-10]. These inhibitors attract particular interest because of their likely participation in the protection of plants from damage caused by insects and phytopathogenic microorganisms [6, 7, 9, 11].
The goal of this study was to purify and characterize three protein inhibitors with molecular weights 20-25 kD from potato tubers (variety Istrinskii) and to determine their N-terminal amino acid sequences.
MATERIALS AND METHODS
The proteins with molecular weights about 20 kD were isolated from potato tuber sap (variety Istrinskii) by ammonium sulfate fractionation and subsequent gel chromatography on a Sephadex G-75 column, as described in [12].
The proteins with molecular weights 20-25 kD in 0.1 M Tris-HCl buffer, pH 8.0, were applied to a DEAE-cellulose column (0.7 × 18 cm) equilibrated with the same buffer. Non-bound proteins were eluted with the same buffer, and after dialysis against 0.05 M acetate buffer, pH 4.3, containing 8 M urea were applied to a CM-cellulose column (0.7 × 18 cm) equilibrated with the same buffer. CM-Cellulose-bound proteins were eluted with a gradient of 0-0.35 M KCl in the starting buffer. Protein fractions were concentrated using ultrafiltration with a PM-10 membrane (Amicon, USA), freed from salts, and lyophilized.
SDS-PAGE in the presence of 0.1% SDS and beta-mercaptoethanol (20% gel) was carried out according to Laemmli [13]. Proteins were stained by 0.1% Coomassie R-250 in 25% ethanol containing 5% formaldehyde.
Protein concentration was determined according to a modified Bradford method [14] using BSA as a standard protein.
The proteins were sequenced using a 470 A gas sequencer (Applied Biosystems, USA) with the standard 02 RPTH program [15]. Amino acid Pth-derivatives were identified by high-performance chromatography. Fractions containing amino acid Pth-derivatives were steamed, dissolved in 20% acetonitrile, and applied to a C18 column (Applied Biosystems). Pth-derivatives were detected using a Cratos 757 flow absorptiometer (USA).
To determine amino acid composition, proteins were oxidized with performic acid [16] and hydrolyzed with 5.7 M HCl at 105°C for 24 h in vacuo. The amino acid content was determined using a Hitachi 855 amino acid analyzer (Japan).
The activity of inhibitors was evaluated from the degree of suppression of the corresponding enzymatic activities (alpha-chymotrypsin, trypsin, human leucocyte elastase, subtilisin, pepsin, cathepsin D, papain, bromelain, and ficin). Proteolytic activity of chymotrypsin and papain was determined toward casein [17], and hemoglobin in the case of pepsin and cathepsin D [18]. Proteinase activity was also evaluated by the rate of hydrolysis of the chromogenic substrates: p-nitroanilides of N-succinyl-L-valyl-L-prolyl-phenylalanine (for chymotrypsin [19]), N-succinyl-L-alanyl-L-alanyl-L-valine (for leucocyte elastase [20]), N-succinyl-glycyl-glycyl-L-phenylalanine (for subtilisin [21]), N,alpha-benzoyl-L-arginine (BAPA, for trypsin [22] and papain [23]), and pyroglutamyl-L-phenylalanyl-L-alanine (for bromelain and ficin [24]).
Trypsin (EC 3.4.21.4), alpha-chymotrypsin (EC 3.4.21.1), human leucocyte elastase (EC 3.4.21.36), subtilisin (EC 3.4.21.14), pepsin (EC 3.4.23.1), cathepsin D (EC 3.4.23.5), crystalline papain (EC 3.4.22.2), bromelain (EC 3.4.22.4), and ficin (EC 3.4.22.3) were used to test the activities of the inhibitors; all preparations were of the highest degree of purification from Sigma (USA). BAPA, Suc-Ala-Ala-Val-pNa, and Suc-Val-Pro-Phe-pNa were from Bachem (Switzerland); Suc-Gly-Gly-Phe-pNa, PGlu-Phe-Ala-pNa, and casein were from NPO Biolar (Latvia); hemoglobin was from Sigma; L-cysteine and EDTA were from Merck (Germany); DEAE-cellulose A-52 and CM-cellulose C-52 were from Serva (Germany); Sephadex G-75 (fine), BSA, reagents for SDS-PAGE, and protein molecular weight markers were from Pharmacia (Sweden).
RESULTS
The proteins precipitated from the potato tuber sap after 75% saturation with ammonium sulfate were subjected to gel filtration. Chymotrypsin inhibitor activity was found in three protein fractions having molecular weights about 40, 20, and 5 kD, but papain inhibitor activity was found only in the protein fraction with molecular weight about 20 kD. This fraction contained at least three protein components with molecular weights 20-25 kD and two additional components with molecular weights 17 and 5 kD by the results of SDS-PAGE from our previous work [12].
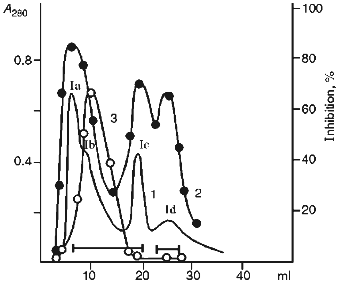
In the first stage, the 20-25 kD protein fractions were separated by ion-exchange chromatography at pH 8.0 using DEAE-cellulose as the sorbent (Fig. 1). Four main protein fractions were obtained after elution with starting buffer. Three of them (Ia, Ic, and Id) inhibited chymotrypsin and only one (Ib) suppressed papain activity.
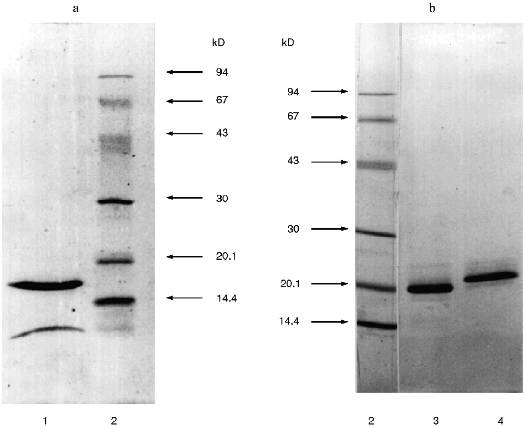
SDS-PAGE under reducing conditions revealed that the Id fraction contained two protein bands with molecular weights 16.5 ± 1 and 4.5 ± 1 kD (Fig. 2a). The protein of the Id fraction migrated as a single band with molecular weight 21 ± 1 kD during SDS-PAGE in the absence of beta-mercaptoethanol. This is evidence that the Id fraction contains a homogeneous protein with molecular weight 21 kD and its molecule is formed by two disulfide-linked polypeptide chains.Fig. 1. Ion-exchange chromatography of the 20-25 kD proteins from potato tubers on a DEAE-cellulose column: Ia-Id) protein fractions; 1) A280; 2) chymotrypsin inhibitor activity; 3) papain inhibitor activity.
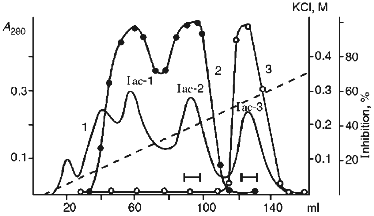
Then pooled fractions Ia-Ic were applied to a CM-cellulose column at pH 4.3 in the presence of 8 M urea. The proteins were eluted with a linear gradient of KCl (0-0.35 M) in the starting buffer. Figure 3 demonstrates that three protein fractions with inhibitor activity were eluted. Iac-1 and Iac-2 fractions were eluted with 0.13 and 0.19 M KCl, respectively, and they inhibited chymotrypsin activity. Iac-3 fraction was eluted with 0.25 M KCl and effectively suppressed papain activity.Fig. 2. SDS-PAGE of proteinase inhibitor preparations from potato tubers: 1) 21-kD protein (Id fraction after ion-exchange chromatography on a DEAE-cellulose column); 3) 22-kD protein (Iac-2 fraction after ion-exchange chromatography on a CM-cellulose column); 4) 23-kD protein (Iac-3 fraction after ion-exchange chromatography on a CM-cellulose column); 2) standard proteins (phosphorylase b, BSA, ovalbumin, carboanhydrase, STI, lactalbumin). The molecular weights of the standard proteins are indicated in kD.
Each of the fractions Iac-2 and Iac-3 contained one protein band with molecular weight 22 ± 1 and 23 ± 1 kD, respectively, according to SDS-PAGE under reducing conditions (Fig. 2b). This is evidence that the protein fractions contain homogenous proteins each of which consists of a single polypeptide chain.Fig. 3. Ion-exchange chromatography on a CM-cellulose column of the pooled Ia-Ic fractions obtained after chromatography on a DEAE-cellulose column: Iac-1, Iac-2, Iac-3) protein fractions; 1) A280; 2) chymotrypsin inhibitor activity; 3) papain inhibitor activity.
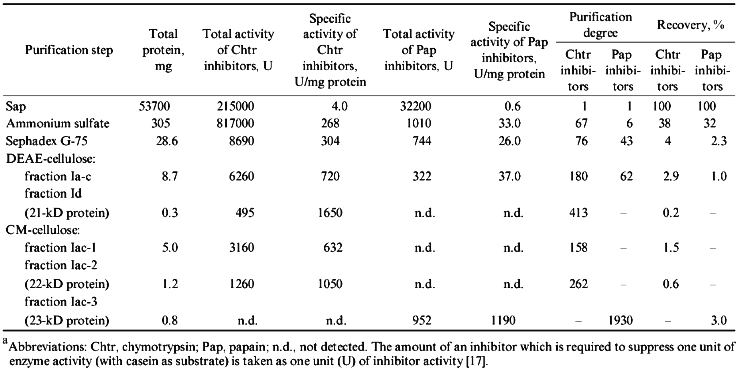
Table 1 summarizes the stages of purification of the protein proteinase inhibitors from the potato tubers. The procedure resulted in 400- and 260-fold purification of the chymotrypsin inhibitors designated as 21- and 22-kD protein, respectively, while the purification of the papain inhibitor (23-kD protein) was 1900-fold.
TABLE 1. Purification of Proteinase
Inhibitors from Potato Tubers (calculated per kg of
tubers)a
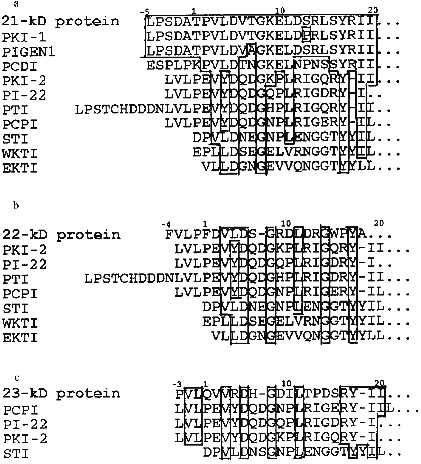
Homogeneity of purified proteins was also proved by the determination of N-terminal amino acid sequences. The N-terminal amino acid sequence of the each protein inhibitor was reconstructed using straight automatic degradation by the Edman procedure (22-25 stages) (Fig. 4). All the isolated proteins have homologous regions in their N-terminal domain: Val8-Leu9-Asp10 and Ile24-Ile25 in the 21-kD protein, Val7-Leu8-Asp9 in the 22-kD protein, and Val6-Arg7-Asp8 and Ile21-Ile22 in the 23-kD protein. This suggests that a related gene family encode this group of proteins in potato tubers. The N-terminal sequences of the isolated proteins include conserved amino acids typical for inhibitors from the Kunitz soybean inhibitor superfamily [25]: Gly13 and Tyr22 in the 21-kD protein, Gly11 in the 22-kD protein, and Gly11 and Tyr20 in the 23-kD protein.
The purified proteins have similar amino acid content with a high level of serine, glycine, and leucine and low amounts of sulfur-containing amino acid residues. There are four cysteine residues in each of these proteins. Considering that after treatment with Ellman reagent [26] free SH-groups were not detected in the proteins, it may be concluded that these proteins include two disulfide bonds. In the case of the 21-kD protein one of the disulfide bonds links protein chains A and B.Fig. 4. N-Terminal amino acid sequences of proteinase inhibitors isolated from potato tubers: 1a) A-chain of the 21-kD protein; 1b) B-chain of the 21-kD protein; 2) 22-kD protein; 3) 23-kD protein. Identical residues are typeset in bold.
Thus, N-terminal sequence analysis together with amino acid content data allows assignment of the isolated proteins to the Kunitz soybean inhibitor superfamily.
Table 2 demonstrates results obtained from studying the activities of purified proteinase inhibitors from potato tubers towards different proteolytic enzymes. The 21-kD protein suppressed human leucocyte elastase with greatest efficiency and this protein is a good inhibitor for trypsin and chymotrypsin. The 21-kD protein does not inhibit activities of subtilisin, plant cysteine proteinases, pepsin, and cathepsin D. The 22-kD protein is an effective trypsin inhibitor; it is less effective towards chymotrypsin and does not inhibit the other studied proteinases. In contrast to these two serine proteinase inhibitors, the 23-kD protein suppresses only cysteine proteinase activity, suppressing activities of papain, ficin, and bromelain to approximately to the same extent.
TABLE 2. Effect of Proteinase Inhibitors
from Potato Tubers on the Activity of Different Proteolytic Enzymes
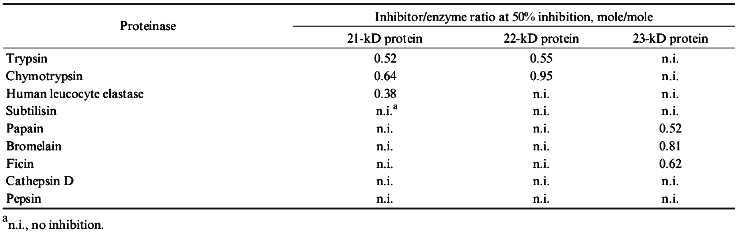
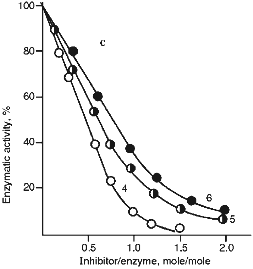
Figure 5 (a and b) demonstrate the effect of increasing concentrations of the 21- and 22-kD inhibitors on the activity of serine proteinases. It is clear from Fig. 5 that when the 21-kD protein interacts with leucocyte elastase, trypsin, and chymotrypsin there is a linear dependence between the degree of inhibition and the quantity of the added inhibitor up to 80-90% inhibition (Fig. 5a), but for the 22-kD protein and trypsin or chymotrypsin linear dependence was observed only to 70% inhibition (Fig. 5b). During the interaction of the 23-kD protein with papain, ficin, and bromelain, linear dependence could be observed also to 70% inhibition (Fig. 5c). We calculated, that one mole of each isolated inhibitor interacted stoichiometrically with one mole of each target proteinase.
Fig. 5. Effect of the 21-kD protein (a), the 22-kD protein (b), and the 23-kD protein (c) on the activity of the target proteinases: 1) human leucocyte elastase; 2) trypsin; 3) chymotrypsin; 4) papain; 5) ficin; 6) bromelain. We used Suc-Ala-Ala-Val-pNa (1), BAPA (2, 4), Suc-Val-Pro-Phe-pNa (3), PGlu-Phe-Ala-pNa (5, 6) as the substrates for determination of enzymatic activity. 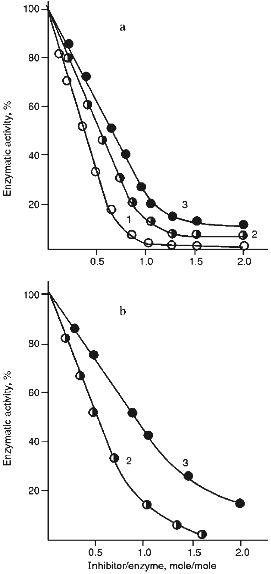
DISCUSSION
Three protein proteinase inhibitors were isolated from potato tubers (variety Istrinskii). Two of them, the 21- and the 22-kD proteins, are serine proteinase inhibitors, while the 23-kD protein is a cysteine proteinase inhibitor. All these proteins have homologous regions in their N-terminal domains. The 22-kD and the 23-kD proteins have one polypeptide chain. The 21-kD protein consists of two disulfide-linked polypeptide chains, the A-chain (16.5 kD) and the B-chain (4.5 kD). It should be mentioned that among the members of the Kunitz soybean inhibitor family there are several previously studied proteins which molecules consisting of two polypeptide chains with molecular weights about 16 and 5 kD. All inhibitors isolated from the seeds of leguminous plants belonging to the Mimosoideae subfamily are from this group of proteins [3, 27, 28]. It is believed that two polypeptide chains were derived from posttranslational restricted proteolysis in the C-terminal region of the protein molecule placed between residues 125-155 (residue numbers are given according to the STI sequence) and encased in a loop formed by a disulfide bond. This part of the molecule is situated on the surface and is readily available for proteinases [29].
Figure 6 shows the N-terminal sequences of the protein inhibitors described in this work and of some other plant proteins, members of the Kunitz soybean inhibitor family. Conserved residues Asp5, Gly8, and Tyr17, typical for the members of the Kunitz soybean inhibitor superfamily, can be found in the sequences of all these proteins. However, the proteins described in this study are similar in their N-terminal regions to some inhibitors previously isolated from potato tubers to a greater extent than to each other. Actually, N-terminal sequences of the 21-kD inhibitor, trypsin inhibitor (PKI-1) [6], and cathepsin D and trypsin inhibitor (PIGEN1) [9] are practically identical. There are differences only between residues 7 and 13: Thr --> Ala (PIGEN1) and Ser --> Pro (PKI-1). However, the 21-kD protein differs from these inhibitors not only in its two-chain structure, but also in its action on the enzymes. Thus, in contrast to PKI-1 [6], the 21-kD protein is a powerful inhibitor of leucocyte elastase, trypsin, and chymotrypsin but it does not inhibit subtilisin. The lack of activity towards aspartyl proteinases (cathepsin D and pepsin) also shows that the 21-kD inhibitor differs from PIGEN1 [9]. N-Terminal sequences of the 22- and 23-kD proteins are similar to the corresponding region of the subtilisin molecule (PKI-2) [6], trypsin inhibitor (PI-22 and PTI) [7, 30], and cysteine proteinase inhibitor (PCPI) [10]. However, they differ from the proteins mentioned above in their specificity of action on proteinases. Thus, the 22-kD protein does not demonstrate activity towards subtilisin and cysteine proteinases, but it is a powerful trypsin inhibitor, as distinct from PKI-2 and PCPI, which were characterized as powerful subtilisin and cathepsin L inhibitors, respectively. The 23-kD protein is a powerful inhibitor of plant cysteine proteinases, distinguishing it from PKI-2, PI-22, and PTI. These facts substantiate the previously suggested idea that the 21-, 22-, and 23-kD proteins are encoded by a family of related genes and make it possible to identify these proteins as members of the potato Kunitz-type proteinase inhibitor family (PKPI) [11]. In this connection the fact that mRNA coding for PKPI accumulates after mechanical damage of the potato tuber is of great interest [11, 31, 32]. This can be indicative of the important part played by these inhibitors in the plant defense from insects and microorganisms. Further investigations are needed to study the participation of these isolated inhibitors in the potato defense system.
This work was supported by the Russian Foundation for Basic Research (project No. 96-04-48079).Fig. 6. A comparison between the N-terminal sequences of the 21-kD (a), the 22-kD (b), and the 23-kD (c) proteins and other proteins from the Kunitz soybean inhibitor family; identical residues are framed; amino acid residues are numbered according to the STI sequence [2]. Inhibitors previously isolated from potato tubers: PKI-1 and PKI-2) trypsin and subtilisin inhibitors [6]; PIGEN1 and PCDI) cathepsin D and trypsin inhibitors [9, 8]; PTI) trypsin inhibitor [30]; PI-22) serine proteinase 22-kD inhibitor [7]; PCPI) cysteine proteinase inhibitor [10]; STI) Kunitz soybean inhibitor [2]; WKTI) inhibitor from the seeds of winged bean [3]; EKTI) trypsin inhibitor from the seeds of Erythrina latissima [4].
LITERATURE CITED
1.Richardson, M. (1991) Plant Biochem.,
5, 259-305.
2.Kim, S. H., Hara, S., Hasa, S., Ikenaka, T., Toda,
H., Kitamura, K., and Kaizuma, N. (1985) J. Biochem.,
98, 435-448.
3.Yamamoto, M., Yara, S., and Ikenaka, T. 91983)
J. Biochem., 94, 849-862.
4.Joubert, F. J., Heussen, C., and Bowdle, B. D.
(1985) J. Biol. Chem., 260, 12948-12953.
5.Svendsen, I., Heijgaard, J., and Chavan, J. K.
(1984) Carlsberg Res. Commun., 51, 43-50.
6.Walsh, T. A., and Twitchell, W. P. (1991) Plant
Physiol., 97, 15-18.
7.Suh, S.-G., Stiekema, W. J., and Hannapel, D. J.
(1991) Planta, 184, 423-430.
8.Mares, M., Meloun, B., Pavlik, M., Kostka, V., and
Baudys, M. (1989) FEBS Lett., 251, 94-98.
9.Srukelj, B., Pungercar, J., Mesko, P.,
Barlic-Magana, D., Gubensek, F., Kregar, I., and Turk, V. (1992)
Biol. Chem. Hoppe-Seyler, 373, 477-482.
10.Krizaj, M., Drobnic-Kosorok, J., Brzin, J.,
Jerata, R., and Turk, V. (1993) FEBS Lett., 333,
15-20.
11.Ishikawa, A., Ohta, S., Matsuoka, K., Hattori,
T., and Nakamura, K. (1994) Plant Cell Physiol., 35,
303-312.
12.Revina, T. A., Valueva, T. A., Ermolova, N. V.,
Kladnitskaya, G. V., and Mosolov, V. V. (1995) Biochemistry
(Moscow), 60, 1844-1842 (Russ.).
13.Laemmli, U. K. (1970) Nature, 227,
680-685.
14.Appenroth, K. J., and Angsten, H. (1987)
Biochem. Physiol. Pflazen, 182, 85-89.
15.Levina, N. B., Slepak, V. Z., Kiselev, O. G.,
Shemyakin, V. V., and Khokhlachev, A. A. (1989) Bioorg. Khim.,
15, 24-31.
16.Hirs, C. H. W. (1956) J. Biol. Chem.,
219, 611-621.
17.Nortrop, D., Kunitz, M., and Herriott, R. (1950)
^Crystalline / Crystal / Enzymes [Russian translation], Izd-vo
Inostrannoi Literatury, Moscow.
18.Ryle, A. P., and Porter, P. R. (1959) Biochem.
J., 73, 75-83.
19.Powers, J. C., Tanaka, T., Harper, J. W.,
Minematsu, Y., Barker, L., Lincoln, D., Grumley, K. V., Fraki, J. K.,
Schechter, N. M., Lazarus, G. G., Nakajima, K., Nakashino, K., Neurath,
H., and Woodbury, R. G. (1985) Biochemistry, 24,
2048-2058.
20.Wenzel, H. R., Engelbrecht, S., Reich, H.,
Mondry, W., and Tschesche, H. (1980) Hoppe-Seyler Z. Physiol.
Chem., 361, 1413-1416.
21.Lyublinskaya, L. A., Yakusheva, L. D., and
Stepanov, V. M. (1977) Bioorg. Khim., 3, 273-279.
22.Kakade, M. L., Simons, N., and Liener, I. L.
(1969) Cereal Chem., 46, 518-526.
23.Barrett, A. J., Kembhavi, A. A., Brown, M. A.,
Kirschke, H., Knight, C. G., Tamai, M., and Hamada, K. (1982)
Biochem. J., 211, 189-195.
24.Filippova, I. Y., Lysogorskaya, E. N., Oksenoit,
E. S., Rudenskaya, G. N., and Stepanov, V. M. (1984) Anal.
Biochem., 143, 293-297.
25.Mosolov, V. V., and Valueva, T. A. ((1993)
Plant Protein Inhibitors of Proteolytic Enzymes [in Russian],
VINITI Publishers, Moscow.
26.Ellman, G. L. (1959) Arch. Biochem.
Biophys., 82, 70-77.
27.Odani, S., Ono, K., and Ikenaka, T. (1979) J.
Biochem., 86, 1795-1805.
28.Souza-Pinto, J. G., Oliva, M. L. V., Sampaio, C.
A. M., Damas, J., Auerswald, E. A., Limaos, E., Fritz, H., and Sampaio,
M. (1996) Immunopharmacology, 33, 330-332.
29.Bode, W., and Huber, R. (1992) Eur. J.
Biochem., 204, 433-451.
30.Yamagishi, K., Mitsumori, C., and Kikuta, Y.
(1991) Plant Mol. Biol., 17, 287-288.
31.Hildmann, T., Ebneth, M., Pena-Cortes, H.,
Sanchez-Serano, J. J., Willmitzer, L., and Prat, S. (1992) Plant
Cell, 4, 1157-1170.
32.Hansen, J. D., and Hannapel, D. J. (1992)
Plant Physiol., 100, 164-168.