Biochemical Mechanisms of Evolution and the Role of Oxygen
V. P. Skulachev
Department of Bioenergetics, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-0338; E-mail: skulach@head.genebee.msu.su
Received August 27, 1998
The concept formulated here presumes the existence of specific mechanisms of evolution that save intermediate (and therefore imperfect) forms of organisms from elimination by natural selection. A change in the life strategy made in situations when the appearance of a new trait worsens, rather than improves, adaptation of the organism to the changing environment can be one of these mechanisms. The concept postulates that, in such cases, K-strategy (relatively low rates of reproduction and activity in general but long life span) is replaced by r-strategy (high activity and reproduction but short life span). A decrease in the life span upon the K --> r transition is suggested to be an unavoidable consequence of an elevation of formation of toxic reactive oxygen species under conditions of increased rates of aerobic metabolism required for the increased life activity. The phenomenon of giantism of transgenic tobacco plants that overproduce a mitochondrial heat shock protein (experiments done by A. Moore) is assumed to be explained by an r --> K transition. On the other hand, a decrease in the life activity and a considerable increase in life span occurring in a nematode upon mutations inhibiting the CoQ biosynthesis (S. Hekimi) might serve as an example of a K --> r transition.
KEY WORDS: evolution, life span, active oxygen species, mHsp70, CoQ
Thus long ago among the giant host-tails
An ancient creature howled with being helpless
When it perceived that on its slimy back
A pair of wings was gradually appearing.
N. Gumilev
The origin and evolution of the living world has been the issue of permanent interest of humankind. The apparently incomprehensible problem of the origin of living organisms, as well as their complexity and perfection, inevitably resulted in religious interpretations. Indeed, is it possible to explain the appearance of an extreme diversity of living organisms without using the notion of the Creator? Random motion in three-dimensional space of a practically unlimited number of variants cannot explain why we are now at the given point of the evolutionary pathway.
Darwin, who introduced the concept of natural selection, limited these motions by performing a kind of transformation of a three-dimensional space into a plane. However, a number of problems remained after Darwin. Natural selection is suitable for quantitative improvement of specific traits of living specimens. It is easy to imagine how selection of random mutations allowed optimization of the length and shape of the forepaw used by biped reptiles for grasping. However, how could this extremity evolve into the wing of an archaeopteryx? This should require the appearance of dozens (or hundreds) of new traits and their preservation in the progeny; each of these traits not only did not give to a specimen any advantage in struggle for existence, but actually should have worsened the previous function of the limb which had already been adapted, through natural selection, to grasping.
Random motion on a plane will hardly provide a solution to the question. It would be better if this plane has a groove that will lead a bead trapped in it to roll directly to the target site. In other words, we should eliminate one more dimension and transform the two-dimensional space into one-dimensional.
This paper will make an attempt to explain, from the biochemist's perspective, how the above limitation imposed by natural selection could be overcome to give rise to a qualitatively new, complex function of a living system.
NATURAL SELECTION COULD HAVE ARRESTED THE PROGRESS OF
EVOLUTION
Within the framework of current concepts, progressive evolution through natural selection should proceed, in a simplest case, through the following chain of events.
1. Occasional mutation or any other change in the genome that affects the performance of a certain gene.
2. Change in the biological function dependent on this gene.
3. Fixation of this change if it gives some advantages for the mutant over specimens carrying no useful mutation (the survival of the most adapted specimens).
This scheme applies perfectly to cases when a mutation is immediately advantageous. However, it does not work when the appearance of useful traits requires subsequent mutations in the same or other gene. In the worst case, the mutation is harmful in first generations of mutants but promises advantage only in the remote future, after accumulation of a considerable number of new mutations. In such a situation, natural selection should be expected to counteract rather than support the progress.
We pointed out in the introduction to this paper that the transformation of the extinct reptile's forepaw into the wing requires such a considerable number of mutations that it is impossible to imagine their simultaneous appearance. Gradual changes of the forepaw transforming it into a wing should have inevitably impaired its grasping function long before the completion of its function in flight. The sieve of natural selection could not be permeable to intermediate forms that were already unable to use their forelimbs in the old function but still could not fly. To resolve this problem, it is important to preserve the intermediate evolutionary forms from the pressure of natural selection.
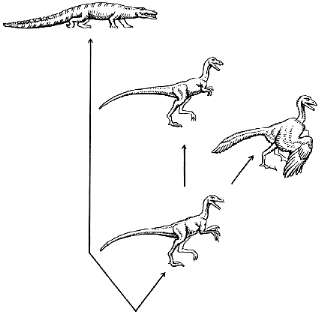
We might suppose that the dinosaur was able to sacrifice the old (grasping) function, which has become less important than the speed of running because of changing environmental conditions. This is consistent with the appearance of tyrannosaurs with their very small forelimbs (Fig. 1). Swinging the buds of their wings, an ancestor of archaeopteryx could increase the speed of its running before acquiring the ability to fly. However, to remain within the framework of the concept of natural selection, we should postulate that each sequential step in the formation of the wing consistently improved the running of the dinosaur. Such a strategy (no backward step) can hardly be called universal or flexible, especially when applied to such a complex task as exploring the ocean of air. The appearance of any new trait would be very difficult if each preceding quantitative change were obliged to give the organism a certain immediate advantage. On the other hand, it is easy to imagine how fast would evolution proceed if special mechanisms of evolution appeared that somehow protected intermediate forms.
Fig. 1. Evolution of reptiles. An extinct quadruped reptile (archaeosaur, not shown) was the ancestor of the present-day crocodile and a biped dinosaur. The evolution of the latter resulted in the appearance of the running tyrannosaur with very small forelimbs (top center) and the flying archaeopteryx (right).
THE r- AND K-STRATEGIES
In 1967, MacArtur and Wilson [1], analyzing the dynamics of sizes of populations, proposed coefficients r and K. In this paper we shall not consider the mathematical sense of these coefficients; rather, we shall use them, after the above authors, to label two strategies used by living creatures in their evolutionary development. The r-strategy involves rapid reproduction and short life span of specimens, whereas K-strategy is a low rate of reproduction with longer life span. The r-strategy is used by populations occurring at points of inflection of their development, when the environment changes; this strategy facilitates the appearance of new traits and expansion over new areas. K-Strategy is characteristic of wealthy populations inhabiting areas they have fought for in the past, under relatively stable environmental conditions. Naturally, the probability of inventing anything new is higher when the rate of reproduction is high and generations change frequently, which means a short life span of specimens (the r-strategy). To resolve the problem of intermediate forms formulated above, it would be desirable to include in the r-strategy, in addition to higher rates of reproduction, a higher rate of the living activity in general (we shall call this quality "the body power"). It seems to be logical that an increase in power should be paid for, and the cost is a decrease in the life span.
If the power of specimens increases in r-strategy, this can compensate for disadvantages of intermediate forms related to the formation of a new function (discussed above). As a result of this, these forms could be able to survive in competition for existence.
Having admitted that the ability to switch between r- and K-strategies is a special biological function, we shall discuss now possible mechanisms of such a switch-over. In the simplest case, there should exist a single gene or a group of coordinated genes whose working mode determines the choice of the strategy.
THE DISCOVERY OF ANTHONY MOORE: A K --> r SHIFT?
In the summer of 1998, Anthony Moore, a British biochemist, made a sensational presentation at the X European Conference on Bioenergetics in Göteborg [2]. His name was not on the initial list of speakers, but he was offered an opportunity to present a nonscheduled talk because of the obviously extraordinary result of his work.
Moore obtained a transgenic tobacco plant with enhanced expression of a single gene which encodes mitochondrial heat shock protein 70 (mHsp70). The overexpression resulted in a 30-fold increase in the level of mHsp70.
The mHsp70 overexpression had striking effects. The transgenic tobacco plant was twofold higher than its normal counterparts (the plants were cultivated under conditions optimal for growth), and there were threefold increases in the mitochondrial dry weight and respiratory rate per mg mitochondrial protein. The rate of photosynthesis was also increased, but to a much smaller extent than the above-mentioned changes. Dr. Annecke Wagner of Holland, who visited Moore's hothouses in Brighten, told me that the transgenic plant, at first glance, gives an impression of belonging to some other species.
What is the mHsp70 protein and what functions is it responsible for?
Heat-shock proteins, a class to which mHsp70 belongs, are primarily known as repair tools correcting other proteins when they adopt a denatured conformation. The polypeptide chain of a native mature protein is folded in a definite shape. On the other hand, polypeptide chain synthesized in ribosomes is unfolded. Its subsequent folding is a necessary stage of formation of a mature protein. Helping it to adopt an appropriately folded shape is a function of heat-shock proteins (the other name, chaperons).
A correct shape of a protein can be lost under unfavorable conditions. Thermal denaturation of proteins is a typical example. Renaturing such proteins is a function of the same heat-shock proteins whose amount increases sharply after heating (hence their name).
Plants contain heat-shock protein species in the cytosol, chloroplasts, and mitochondria; mHsp70 is found in mitochondria only. In addition to being involved in maturation of 15 proteins synthesized in mitochondria and their renaturing, mHsp70 plays a major role in mitochondrial import of proteins whose synthesis occurs in the cytosol (they form the majority of mitochondrial proteins, approximately 500 species). This is a dual role. Proteins are imported by mitochondria in unfolded forms. As soon as the N-end of the transported protein appears inside the mitochondrion, it binds to mHsp70, which causes (by a still unknown mechanism) traction of the remaining part of the polypeptide into the mitochondrion. In addition, mHsp70 ensures correct folding of the initially unfolded polypeptide chain of the imported protein [3-6].
In addition to the above-listed functions (correct folding of newly synthesized and imported proteins, acceleration of import of proteins, and renaturing of denatured mitochondrial proteins), yeast mHsp70 were found to display a property whose significance remains unexplained: together with another protein of 50 kD, mHsp70 forms mitochondrial endonuclease Sce1, which cleaves mitochondrial DNA at some specific sites [7].
It is unclear which of these properties of mHsp70 is responsible for above mentioned striking changes in the phenotype of the transgenic tobacco plant. However, the simplest explanation is probably as follows. 1) A strong increase in the mHsp70 level accelerates the import and maturation of mitochondrial proteins, including enzymes involved in respiration and oxidative phosphorylation and responsible for ATP synthesis. (Moore [2] reported that the rate of mitochondrial import of proteins was 3.5-fold higher in the transgenic plant than in normal tobacco plants.) 2) High respiratory rate found in Moore's experiments results in formation of extra amounts of ATP, thereby increasing the supply of energy to the plant cells. 3) Increased energy supply stimulates the growth of the plant.
Having agreed with this logic of events, we come to the question of why normal level of mHsp70 is so low that it limits the rates of mitochondrial respiration and of the coupled phosphorylation. One possible explanation is that the maximal activity of mitochondrial respiration systems has a dangerous side effect. It is known that approximately 98% of oxygen consumed by respiring mitochondria is converted to water, whereas the remaining 2% results in superoxide anion (O2) through side reactions occurring in the initial and middle spans of the respiratory chain. Superoxide is a precursor of hydrogen peroxide (H2O2), which is further converted to hydroxyl radical (OH). The latter is the most potent oxidant, capable of destroying any substance in living cells (Fig. 2). An increase in the flow of electrons in the respiratory chain from respiratory substrates to oxygen inevitably increases the production of O2 and then OH. One of the most widely accepted theories of aging [8-15] explains aging by production of reactive oxygen species (ROS) such as OH. Therefore, high levels of mHsp70 cause an increase in energy supply and growth acceleration but can shorten the life span of the specimen because of production of toxic oxygen derivatives.
On the other hand, one can imagine a more complex scenario for the mHsp70 superproducer, which results in the same effect--growth acceleration and an increase in the activity of the organism accompanied by a decrease in its life span. The key role in this relationship might be played by the interaction of mHsp70 with the 50-kD subunit of mitochondrial endonuclease Sce1.Fig. 2. Pathways of electron transport in mitochondrial respiratory chains. Most electrons (e-) abstracted from respiratory substrates are transported through NAD, CoQ, and cytochrome c to oxygen, resulting in the formation of water (solid arrows). However, a fraction of electrons (usually, not more than 2%) is abstracted by oxygen at the initial and middle spans of the chain. The product of this reaction is superoxide (O2), which is converted to hydrogen peroxide (H2O2) and hydroxyl radical (OH), the most active chemical oxidant (dashed arrows).
Most probably, activity of this endonuclease normally is somehow arrested to avoid destruction of mitochondrial DNA. mHsp70 may be a regulatory subunit of Sce1 because its active site is located on the other subunit (50 kD), which, however, does not cleave DNA in the absence of mHsp70 [7]. Apparently, the activating effect of mHsp70 is blocked in intact mitochondria. The block is removed upon destruction of mitochondria (it is after this procedure that the endonuclease activity of Sce1 is observed [7]).
Admit for a moment that denatured proteins bind to mHsp70 and such a binding results in activation of endonuclease inside mitochondria. If it is the case, an increase in the level of denatured proteins in a mitochondrion will result in cleavage of its DNA and degradation of this mitochondrion. This can serve as a mechanism of scavenging those mitochondria that contain high levels of denatured proteins from the total mitochondrial population. Such a process (I call it "mitoptosis" [16]) can lead to programmed cell death (apoptosis) when denatured proteins are accumulated in a considerable number of mitochondria and mitoptosis occurs on a mass scale. A key factor here may be that mitoptosis is accompanied by liberation of mitochondrial proteins into the cytosol, and some of these proteins (cytochrome c, apoptosis-inducing factor AIF, and caspase 3) activate apoptosis [17-22]. Moreover, some other event might be involved in the mHsp70-linked apoptosis. In this connection, an interesting finding is that heat shock causes the appearance of mHsp70 in the cell nucleus [7].
One function of apoptosis is to remove from the cellular population those cells which have acquired hazardous properties. One important defense of the organism against viral infection is to eliminate by apoptosis infected cells and the closest neighboring cells which are the most frequent targets of infection. The same strategy is probably used by tissues resisting malignant transformation [23].
A strong increase in the level of mHsp70 should result in a situation when mitochondrial endonuclease 50-kD subunit always deals with free mHsp70 rather than with mHsp70 bound to denatured proteins. In the scheme discussed above, this should be expected to prevent both mitoptosis and apoptosis. An easily imaginable situation is that a certain increase in the level of mHsp70 weakens antiviral and anticancer defenses but does not cause their total breakdown. At early steps, this would increase the instantaneous life potential (because fewer mitochondria and cells commit suicide) but reduce the life span (a greater probability of death of viral infection or cancer).
In both variants discussed above, overproduction of mHsp70 appears to be a molecular mechanism of switching the living system from K-strategy to r-strategy. Within a given time interval, this would certainly give certain advantages to the organism at the cost of a decrease in its life span.
This situation is similar to a known story. In the beginning of World War II, A. S. Yakovlev, a famous Russian airplane constructor, decided to considerably simplify the design of engines used in fighter planes. This strongly increased the rate of their production but decreased their longevity to dozens of hours. This permitted Russia to build many fighters over a limited time interval and achieve dominance over the Luftwaffe. At the same time, Britain continued the production of engines for their fighters at Rolls-Royce plants which guaranteed 1200 h of flight; the result was the Coventry tragedy. The decision made by Yakovlev was determined by simple reasoning: in that war, the life span of a fighter plane was 10-20 h. Clearly, his idea is a typical example of r-strategy. It can be a perfect solution in critical war periods but becomes unusable after establishment of peace.
Let us, however, return to Moore's experiments. Dr. Moore was probably lucky to activate a "strategic switch" and elicit the K --> r transition. Importantly, Moore's effect was found to be specific to mHsp70. Overproduction of other, nonmitochondrial Hsp is known to be without such dramatic effects as in the case with mHsp70. An increase in the production of the complex III non-heme iron protein, operating in the middle part of the respiratory chain, also did not affect the plant growth. Moore wrote to me in a recent letter that an effect similar to that observed in tobacco had already been reproduced in his experiments with transgenic mice overproducing mHsp70.
The latter finding suggests that Moore has encountered a feature of general biological significance. It is also clear that the necessary effects are mediated by intracellular (more precisely, intramitochondrial) events rather than regulatory processes acting at a level of tissues or whole organism, such as hormonal regulation. In that respect the above effects clearly differ from giantism caused by growth hormones. These hormones are entirely different in plants and animals and have nothing to do with mitochondria and respiratory enzymes. On the other hand, the actions of mHsp70 and growth hormones have a common feature. In both cases, an increase in production of a single substance causes strong stimulation of body growth. In fact, the organisms are ready to respond to an increase in mHsp70 level by strong stimulation of the growth rate. This indicates that the response to overproduction of mHsp70 is a physiological phenomenon rather than an artefact.
A MUTANT NEMATODE Caenorhabditis elegans: AN r --> K SHIFT?
Now we shall discuss a relatively small object, Caenorhabditis elegans, which consists of only 945 cells whose generation and destiny have already been followed by embryologists. The genome of this worm has several genes whose mutations increase the life span of the specimen. S. Hekimi, a Canadian biologist, reported [24] that simultaneous switch-off of two such genes caused a more than fivefold increase in life span (Fig. 3). Life spans of both larvae and sexually mature adult forms increased with a decrease in reproductive ability and food consumption and an increase in susceptibility to damage by hydrogen peroxide or conditions facilitating superoxide formation (e.g., ultraviolet irradiation, treatment with methyl viologen or high concentrations of O2) as well as by high temperature. In 1997, this author found a gene in the human genome that is very similar to one of the two worm genes [25]. A similar gene was also found in yeast, and some information is already available on the function of the protein encoded by this gene: it plays a major role in switching metabolism from oxygen-deficient (anaerobic) to oxygenated (aerobic) mode. In particular, this protein is necessary for derepression of the gene coding for an enzyme responsible for the synthesis of carbohydrates and, which is especially important in this context for the synthesis of CoQ [25]. It is well known that semiquinone form of CoQ readily reduces oxygen to superoxide [26]. Other studies previously showed that mutation in the gene coding for an enzyme involved in the CoQ synthesis causes a strong increase in the survival rate of a yeast mutant deficient in superoxide dismutase, an important antioxidant enzyme [27].
Previous studies of Drosophila and yeast found genes that increase the life span (although not so dramatically as in the worm) and simultaneously make the organism and cells more resistant to oxidative stress, thermal treatment, and starvation. S. Murakami and T. E. Johnson have proposed calling these genes "gerontogenes" [28].Fig. 3. Life span of nematode C. elegans: 1) control; 2) mutation 1; 3) mutation 2; 4) mutations 1 and 2 (from [24]).
Returning to experiments with nematodes, we can conclude that the double mutant obtained by Hekimi displays traits which are opposite to those of Moore's transgenic plant, i.e., the former had lower rates of growth and metabolism (mitochondrial respiration was probably the most suppressed process, considering a possible block of biosynthesis of CoQ, a key component of the respiratory chain; see above and Fig. 2). In other words, the nematode was forced to shift to K-strategy (Hekimi), whereas tobacco was shifted to r-strategy (Moore).
A POSSIBLE MECHANISM OF SWITCHING BETWEEN STRATEGIES: ROLE OF
OXYGEN
The question to be answered is how the above strategies are switched over under natural conditions. It is tempting to speculate that the central role in this process belongs to the same reactive oxygen species.
Living cells permanently balance the processes of formation and inactivation of ROS, so the ROS level remains low but always above zero [23]. Adverse environmental conditions initiate attempts of organisms to resist the environment that became more aggressive. As a result, energy expenditures increase, and therefore respiration, the main energy-supplying process, is activated. Activation of respiration increases the yield of ROS, whereas high level of ROS accelerates aging and decreases life span. This results in the formation of active, strong forms of living species, a kind of "superspecimens"; however, they will die early of cancer, viral infections, or senescence.
The relationship between ROS and fertility is less understood. However, there is evidence that ROS increase the rate of cell divisions, at least in some cell types [23, 29-35]. This effect is mediated by special proteins (NF-kB, c-jun, p21ras, p44MAPK, and c-fos).
There is no doubt that ROS are the main mutagen in aerobic cells. Stimulated mutagenesis not only decreases the life span but also increases the genetic diversity of new generations. Certain mutations resulting from attacks of ROS on DNA are useful and can be preserved by natural selection. Thus, the organism can adapt to changes in environmental conditions; its enhanced activity becomes no longer necessary, and its respiratory rate decreases, resulting in lower level of ROS. A decrease in ROS level will decrease the rate of reproduction and increase the life span. This means a shift in strategy from r to K.
The above-discussed simplistic (linear) scheme, if it actually works, should obligatorily contain a system of feedback loops that amplify signals leading to switch-over between strategies. At the cellular level, this can be mediated by a known phenomenon of accelerated shortening of terminal DNA fragments (telomeres) caused by ROS [36] whose level increases upon a K --> r shift. This should decrease the maximal possible number of cell divisions (Hayflick's limit) and accelerate cell aging [37, 38].
Induction of Hsp in response to an increase in the level of denatured proteins can serve as an additional example of such a mode of signal amplification [39-41]. The rate of protein denaturation should be expected to increase under unfavorable conditions because of higher levels of ROS, whereas simultaneous increases in Hsp concentrations will lead to the effect described by Moore.
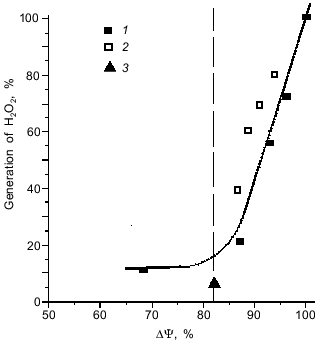
Interestingly, a decrease in the level of ROS upon a decrease in the activity of the organism (r --> K shift) is a limited process. Our experiments showed [42] that the rate of generation of ROS by the respiratory chain displays a threshold dependence on the magnitude of the membrane potential, an intermediate of the mitochondrial ATP synthesis. When membrane potential increases above a certain critical level, the production of ROS increases abruptly (Fig. 4). This threshold level can be surpassed under the work-to-rest transition, when ATP expenditure strongly decreases and the respiratory rate is limited by availability of ADP. In other words, not only increased activity, but also idleness is harmful to health because both entail accumulation of toxic oxygen derivatives.
Fig. 4. Threshold dependence of the formation of hydrogen peroxide by mitochondria on the magnitude of membrane potential (DeltaPsi). The membrane potential was reduced by addition of: 1) SF6847, an uncoupler that stimulates respiration through an increase in proton conductance of the mitochondrial membrane; 2) malonate, which inhibits supply of the respiratory chain with electrons; and 3) ADP, which stimulates membrane potential utilization and, hence, respiration (from [42]).
HUMANS AND EVOLUTIONARY MECHANISMS
Above we have discussed an example of a possible mechanism that can accelerate biological evolution. This mechanism could involve a change in the life strategy of a population: worsening of life conditions stimulates the appearance of more potent, actively respiring and reproducing specimens having a shorter life span (r-strategy). The appearance by natural selection of more adapted organisms decreases their activity, respiratory rate, and reproduction rate, but increases the life span (K-strategy). At the biochemical level, the key role in changes in the ratio between r and K strategies can belong to ROS.
In the above-discussed concept, any currently existing species uses one of these two strategies or exists at the stage of change between these strategies.
Humans cannot be an exception to this rule if it applies to all living species. However, humans, who began their development primarily as a usual biological species, have now reached a stage when laws of biological evolution are no longer essential. When we want to fly, we build a plane rather than waiting millions of years until wings grow out of our back. For humans, specific mechanisms of evolution, if they actually exist, are not more than an atavism, which can be far from being harmless. For example, we have pointed out above that a shift toward r-strategy can involve a decrease in the life span, an earlier susceptibility to malignancies and senescence. There is hope that having studied the mechanisms that switch between r and K strategies and having managed to control these mechanisms, we can put an end to such a dangerous atavism and prolong the time of healthy life of humans.
Obviously, we should be extremely cautious in designing such projects and follow the main wisdom of medicine "Do no harm". We should first obtain all possible information from experiments with plants and animals. These studies can per se have a great effect on agricultural production. For instance, transgenic animals and plants overproducing mHsp70 should be expected to grow faster than normal specimens. Food products prepared from the mHsp70 overproducers can be free of unfavorable side effects of products prepared from animals treated with growth hormones. In contrast to growth hormones that act from outside the cell, mHsp70 works inside mitochondria where it is transported from the cytosol in the form of a longer precursor protein that carries an additional (leader) sequence of 46 amino acid residues [43, 44]. Therefore, mHsp70 ingested with food, in contrast to hormones, is harmless because it cannot reach its target site. First, cell membranes are usually impermeable to hydrophilic proteins such as mHsp70. Second, even having appeared inside a cell, mHsp70 will not reach the mitochondrial matrix because the mature protein is deprived of its leader sequence. A precursor of mHsp70 is always contained in cells in much smaller amounts relative to mature mHsp70. In addition, the precursor, like mHsp70, will most probably be unable to cross the cell membrane. This is why excess mHsp70 in nutrition will hardly be hazardous to humans.
In conclusion, it should be stressed that the above-considered biological tool that promotes evolution is only a particular example taken to illustrate the current state of the problem. It seems probable that living nature has invented other means limiting the random search for a variant of further development that will be optimal under the given conditions. In humans, any of these mechanisms look like an inborn defect that should be corrected, because traits favorable for evolution of the species can be unfavorable for the specimen.
I am grateful to D. A. Knorre who drew my attention to works done by MacArtur and Wilson. I am also grateful to A. N. Khokhlov, Yu. A. Labas, S. P. Maslov, A. Moore, A. S. Rautian, A. S. Severtsev, F. F. Severin, M. V. Skulachev, and M. Yu. Sherman for valuable advice, discussion, and constructive criticism. A popular variant of this paper has been submitted to the journal Priroda upon request of the Editorial Board.
REFERENCES
1.MacArtur, R. H., and Wilson, E. O. (1967) The
Theory of Island Biogeography, Princeton University Press,
Princeton.
2.Moore, A. (1998) Report at 10 EBEC,
Göteborg.
3.Pfanner, N., Craig, E. A., and Meijer, M. (1994)
Trends Biochem. Sci., 19, 368-372.
4.Schneider, H.-C., Berthold, J., Bauer, M. F.,
Dietmeier, K., Guiard, B., Brunner, M., and Neuper, W. (1994)
Nature, 371, 768-774.
5.Horst, M., Azem, A., Schatz, G., and Glick, B. S.
(1997) Biochim. Biophys. Acta, 1318, 71-78.
6.Heyrovska, N., Frydman, J., Höhfeld, J., and
Hartl, F. U. (1998) J. Biol. Chem., 379, 301-309.
7.Morishima, N., Nakagawa, K., Yamamoto, E., and
Shibata, T. (1990) J. Biol. Chem., 265, 15189-15197.
8.Harman, D. (1956) J. Gerontol., 11,
298-306.
9.Miquel, J., and Fleming, J. (1986) Mod. Aging
Res., 8, 51-74.
10.Kitagawa, T., Suganuma, N., Nawa, A., Kikkava,
F., Tanaka, M., Ozawa, T., and Tomoda, Y. (1993) Biol. Reprod.,
49, 730-736.
11.Shigenaga, M. K., Hagen, T. M., and Ames, B. N.
(1994) Proc. Natl. Acad. Sci. USA, 101, 10771-10778.
12.Luft, R. (1995) Biochim. Biophys. Acta,
1271, 1-6.
13.Papa, S., and Skulachev, V. P. (1997) Mol.
Cell. Biochem., 174, 305-319.
14.Ozawa, T. (1997) Biosci. Rep., 17,
237-250.
15.Skulachev, V. P. (1997) Biochemistry
(Moscow), 62, 1394-1399 (Russ.).
16.Skulachev, V. P. (1998) Biochim. Biophys.
Acta, 1363, 100-124.
17.Zamzami, N., Susin, S. A., Marchetti, P., Hirsch,
T., Gomez-Monterrey, M., Castedo, M., and Kroemer, G. (1996) J. Exp.
Med., 183, 1533-1544.
18.Skulachev, V. P. (1996) FEBS Lett.,
397, 7-10.
19.Liu, X., Naekyung, C., Yang, J., Jemmerson, R.,
and Wang, X. (1996) Cell, 86, 147-157.
20.Yang, J., Liu, X., Bhalla, K., Kim, C. N.,
Ibrado, A. M., Cai, J., Peng, T.-I., Jones, D. P., and Wang, X. (1997)
Science, 275, 1129-1132.
21.Kluck, R. M., Bossy-Wetzel, E., Green, D. R., and
Newmeyer, D. D. (1997) Science, 275, 1132-1136.
22.Skulachev, V. P. (1998) FEBS Lett.,
423, 275-280.
23.Skulachev, V. P. (1999) in Bioscience-2000
(Pasternak, C. A., ed.) World Sci. Publ., Singapore-London
(accepted).
24.Lakowski, B., and Hekimi, S. (1996)
Science, 272, 1010-1013.
25.Ewbank, J. J., Barnes, T. M., Lakowski, B.,
Lussier, M., Bussey, H., and Hekimi, S. (1997) Science,
275, 980-983.
26.Skulachev, V. P. (1996) Quart. Rev.
Biophys., 29, 169-206.
27.Longo, V. D., Gralla, E. B., and Valentina, J. S.
(1996) J. Biol. Chem., 271, 12275-12280.
28.Murakami, S., and Johnson, T. E. (1996)
Genetics, 143, 1207-1218.
29.Rao, G. N., and Berk, B. C. (1992) Circ.
Res., 70, 593-599.
30.Nashio, E., and Watanabe, Y. (1997) Biochem.
Biophys. Res. Commun., 232, 1-4.
31.Murrell, G., Francis, M., and Bromley, L. (1990)
Biochem. J., 265, 659-665.
32.Dypbukt, J. M., Ankarcrona, M., Burkitt, M.,
Sjoholm, A., Strom, K., Orrenius, S., and Nicotera, P. (1994) J.
Biol. Chem., 269, 30553-30560.
33.Bhunia, A. K., Han, H., Snowden, A., and
Chatterjee, S. (1997) J. Biol. Chem., 272,
15642-15649.
34.Lee, S.-L., Wang, W.-W., and Fanburg, B. L.
(1998) Free Rad. Biol. Med., 24, 855-858.
35.Ioshikawa, Y., Yokoo, T., and Kitamura, M. (1997)
Biochem. Biophys. Res. Commun., 240, 496-501.
36.Von Zglinicki, T., Sarezki, G., Docke, W., and
Lotze, C. (1995) Exp. Cell Res., 220, 186-193.
37.Olovnikov, A. M. (1971) Dokl. Akad. Nauk
SSSR, 201, 1496-1498.
38.Bodnar, A. G., Ouellette, M., Frolkis, M., Holt,
S. E., Chiu, C.-P., Morin, G. B., Harley, C. B., Shay, J. W.,
Lichtsteiner, S., and Wright, W. E. (1998) Science, 279,
439-352.
39.Ritossa, H. (1962) Experimentia,
18, 571-573.
40.Greider, C. W., and Blackburn, E. H. (1985)
Cell, 43, 405-413.
41.Liu, A. Y.-C., Lee, Y.-K, Manalo, D., and Huang,
L. E. (1996) in Stress-Induced Cellular Responses (Felge,
U., Morimoto, R. I., Jahara, I., and Polfa, B., eds.) Birkhayser
Verlag, Basel, pp. 393-408.
42.Korshunov, S. S., Skulachev, V. P., and Starkov,
A. A. (1997) FEBS Lett., 416, 15-18.
43.Singh, B., Soltys, B. J., Wu, Z. C., Patel, H.
V., Freeman, K. B., and Gupta, R. S. (1997) Exp. Cell Res.,
234, 205-216.
44.Bhattacharyya, T., Karnezis, A. N., Murphy, S.
P., Hoang, T., Freeman, B. C., Phillips, B., and Morimoto, R. I. (1995)
J. Biol. Chem., 270, 1705-1710.