Artificial Photosynthesis: a Model Light-Dependent Proton Pump without Protein Generates Membrane Potential Used by H+-ATP-Synthase for ATP Synthesis
I. I. Severina1 and V. P. Skulachev2*
1Department of Physico-Chemical Biology, School of Biology, Lomonosov Moscow State University, Moscow, 119899 Russia2Department of Bioenergetics, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-0338; E-mail: skulach@head.genebee.msu.su
* To whom correspondence should be addressed.
Received August 27, 1998
In 1997-1998, G. Steinberg-Yfrach, J.-L. Rigaud, and their coauthors published two works that mark a serious breakthrough in creating a system of artificial photosynthesis [1, 2]. These groups in Arizona and Paris succeeded in obtaining light-induced transmembrane charge separation in liposomes and transforming the energy of light into proton potential through interactions of derivatives of porphyrin and quinone [1]. The same process was then demonstrated in experiments with proteoliposomes that contain, in addition to the porphyrin pigment and quinone, a purified H+-ATP-synthase from chloroplasts (the complex CF0F1). This system catalyzed the photosynthesis of ATP from ADP and phosphate [2].
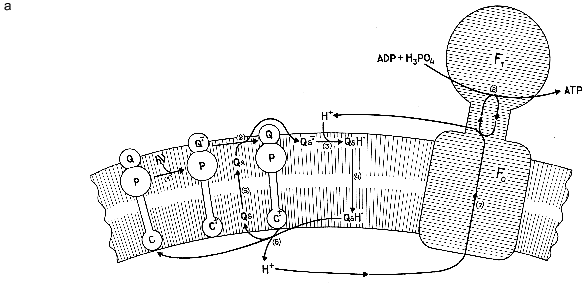
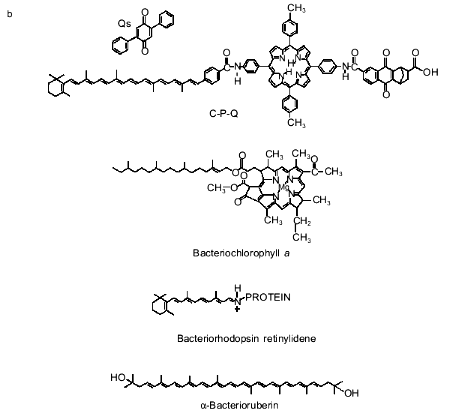
The pigment role was played by a molecule consisting of three moieties, namely, carotenoid, porphyrin derivative, and substituted naphthoquinone (C-P-Q, the figure (b)). This substance was added to liposomes or proteoliposomes containing CF0F1. Liposomes were prepared from a mixture of egg phosphatidylcholine and phosphatidic acid (10:1), cholesterol (20 mole %), and 2,5-diphenylbenzoquinone (Qs, the figure (b)). C-P-Q was incorporated in the membrane by its hydrophobic (carotenoid) "tail", whereas the hydrophilic "body" of the molecule (porphyrin and naphthoquinone carrying a carboxyl group) remained near the outer face of the membrane. The carotenoid, which is a stretched, rigid structure (a "stick" of four isoprenoid residues with an ionone ring on one end) was oriented normal to the membrane plane, so the ionone ring was positioned in the vicinity of the inner face of the membrane. The inverse orientation of C-P-Q (ionone ring outside) was impossible because the pigment molecule should then be dipped in the membrane by its hydrophilic body, leaving the hydrophobic tail in water. Each liposome contained approximately 1000 C-P-Q molecules and 500 Qs molecules.
Illumination of these proteoliposomes with laser light at lambda = 633 nm under anaerobic conditions induced generation of a transmembrane difference of H+ potentials (DeltaµH+). Adding ADP under these conditions induced ATP synthesis rate of which was linear for at least 1 h. The process was completely blocked by a protonophore uncoupler or tentoxin, an inhibitor of H+-ATP-synthase. The quantum yield of phosphorylation reached 7%, and the efficiency coefficient was 4%. The rate of photophosphorylation was 7 moles ATP per mole H+-ATP-synthase per second, which was higher than that measured in proteoliposomes containing the protein complex of photosystem I and H+-ATP-synthase [3] and similar to the rate of ATP synthesis in proteoliposomes with bacteriorhodopsin and H+-ATP-synthetase [4].Model photosynthetic system of Steinberg-Yfrach and coauthors [2] (a) and formulae of certain natural and synthetic pigments related to photosynthesis (b).
The authors suggested that a possible mechanism of this reaction is described by the scheme shown in the figure (a). Absorption of one light quantum induces an intramolecular shift of electrons from the ionone ring of the carotenoid toward naphthoquinone (step 1), thereby facilitating oxidation of the latter by quinone Qs (step 2). After being reduced, Qs abstracts a proton from the incubation medium (step 3). The QsH produced in this process diffuses along its concentration gradient to the opposite side of the membrane (step 4), where it is oxidized (step 5) by the ionone ring of the carotenoid that has lost an electron in the first step of the process. The oxidized quinone returns to its starting position near the outer face of the membrane (step 6). The proton released into the internal aqueous space of the proteoliposome at step 5 is returned to the external water through the subcomplex of F0 (membrane part of H+-ATP-synthase) (step 7) coupled to ATP synthesis in the catalytic subcomplex F1 (step 8).
The same figure shows the formula of chlorophyll a. It is evident that chlorophyll and C-P-Q have several common and distinctive features. Both compounds contain a tetrapyrrole chromophore group (porphyrin in C-P-Q and Mg-porphyrin in chlorophyll) and a tetraisoprene residue (phytol in chlorophyll and carotenoid in C-P-Q). However, chlorophyll lacks several cyclic groups, i.e., ionone ring, naphthoquinone, and five benzene rings found in the C-P-Q molecule. C-P-Q has a free carboxyl group. Chlorophyll has two carboxyl groups, but both are involved in ester bonds (with phytol and methanol). Finally, the tetraisoprene ring of chlorophyll has only one double bond, whereas C-P-Q has four.
An interesting hypothesis is that C-P-Q is an archetype of a primary pigment used by ancestors of modern photosynthetic bacteria. Storage of light energy by means of C-P-Q looks simpler than, for example, in modern purple bacteria, whose transmembrane electron transport is mediated by a complex consisting of: a) bacteriochlorophyll dimer; b) bacteriochlorophyll monomer; c) bacteriopheophytin (a magnesium-free analog of bacteriochlorophyll); d) tightly bound ubiquinones and menaquinones; and e) three to four protein subunits [5].
A protein is also required for the bacteriorhodopsin light-dependent H+ pump, which is simpler but less efficient than the chlorophyll--protein complex. Interestingly, the chromophore in bacteriorhodopsin is retinal, a member of the carotenoid family, linked to the protein through a Schiff base (the figure (b)). Remember that also in C-P-Q a carotenoid residue is linked to the porphyrin moiety of the molecule through a Schiff base. In bacteriorhodopsin, like in C-P-Q, absorption of a light quantum shifts the electron density along the chain of double bonds in the carotenoid chromophore. However, this does not result in the transfer of an electron across the membrane; rather, this causes trans-cis-isomerization of retinal followed by conformational change in the protein and transmembrane transport of a proton [6].
In light of this, evolution of the photosynthetic system may have proceeded as follows.
1. Opacification of the atmosphere, which became impermeable to ultraviolet light, made it necessary to use visible light and abandon the "ultraviolet" photosynthesis based, in our opinion, on the use of the adenine residue of adenosine diphosphate as a chromophore and ATP as a high-energy product [7]. The chromophore that absorbed visible light was a carotenoid derivative like bacterioruberin (the figure (b)), which is sufficiently long to cross the membrane and ensure charge separation in response to absorption of a photon. The asymmetry of orientation of the chromophore in the membrane was due to the presence of a hydrophilic (for example, carboxyl) group on one end of the carotenoid.
2. The first improvement of the chromophore was that the porphyrin group was linked to the carotenoid, which increased the coefficient of light absorption by the pigment molecule and shifted the absorption maximum toward long-wave quanta that penetrate the sea water to a greater depth. The hydrophilicity was again imparted by the carboxyl group linked to porphyrin; however, at that time this group was not yet methylated and remained free. The pigment was formed inside the cell, entered the cell membrane, and dissolved in it by its hydrophobic carotenoid residue. As a result of this, the ionone ring was positioned in the vicinity of the outer face of the membrane, whereas the carboxyl group of porphyrin was found on its inner side. Thus, photoinduced electron transfer occurred in the direction opposite to that found by Steinberg-Yfrach and their colleagues; however, it was consistent with what occurs in modern bacteria (the cell interior is positive). The cause is trivial: Steinberg-Yfrach et al. added the chromophore from outside the liposome, whereas the cell synthesized it inside.
3. Further improvement of this system consisted in appearance of a chain of chromophores across the membrane; the chromophores were tightly fixed on the protein, which served as a support. Such a structure allowed a strong decrease in the life span of the excited state of the pigment. Previously, this time should have been long enough for the quinone to collide in the membrane with the ionone ring that acquired excessive electron density. Now, free quinone was not obliged to interact with the excited pigment because the function of charge separation was given to other molecules fixed rigidly in the immediate vicinity of each other at distances optimal for the performance of their functions. This not only prevented a back shift of the electron after quantum absorption, but considerably decreased the probability of oxidation of the excited pigment by molecular oxygen. (Such an oxidation is a permanent threat to the photosynthetic system of aerobic bacteria that use the energy of light. For this reason Steinberg-Yfrach and coauthors conducted their experiments under stringent anaerobic conditions [2].)
In the new structure where chlorophyll was dipped in the membrane, the carboxylic group became more a disadvantage than a necessary component, and thus it was then methylated. The carotenoid residue lost its ionone ring, and all double bonds except one were reduced. As a result of this, the residue acquired flexibility necessary for being compacted in a special pocket inside the protein molecule.
All this applies to evolution of photosynthesis in bacteria. The same process underwent a different type of evolution in archaei. There, a carotenoid pigment remained, but transmembrane electron transfer within a single long molecule was rejected. It was replaced by proton transfer by means of light-induced changes in the conformations of the chromophore and then the protein.
In conclusion, we should stress that, in addition to purely theoretical interest, the model of Steinberg-Yfrach et al. has a definite practical significance. It can be very useful for, e.g., regeneration of ATP in cell-free protein synthesis systems.
REFERENCES
1.Steinberg-Yfrach, G., Lindell, P. A., Hung, S.-C.,
Moore, A. L., Gust, D., and Moore, T. A. (1997) Nature,
385, 239-241.
2.Steinberg-Yfrach, G., Rigaud, J.-L., Durantini, E.
N., Moore, A. L., Gust, D., and Moore, T. A. (1998) Nature,
392, 479-482.
3.Cladera, J., Rigaud, J.-L., Bottin, H., and Dunach,
M. (1996) J. Bioenerg. Biomembr., 28, 503-515.
4.Pitard, B., Richard, P., Dunach, M., and Rigaud,
J.-L. (1996) Eur. J. Biochem., 235, 779-788.
5.Deisenhofer, J., Epp, O., Miki, K., Huber, R., and
Michel, H. (1985) Nature, 318, 618-624.
6.Skulachev, V. P. (1993) Quart. Rev.
Biophys., 26, 177-199.
7.Skulachev, V. P. (1994) Antonie van
Leuwenhoek, 65, 271-284.