Kinetics of the Interaction of Rabbit Skeletal Muscle Phosphorylase Kinase with Glycogen
I. E. Andreeva, V. F. Makeeva, B. I. Kurganov*, N. A. Chebotareva, and N. B. Livanova
Bach Institute of Biochemistry, Russian Academy of Sciences, Leninskii pr. 33, Moscow, 117071 Russia; fax: (095) 954-2732; E-mail: inbio@glas.apc.org* To whom correspondence should be addressed.
Received June 16, 1998; Revision received September 21, 1998
The kinetics of the interaction of rabbit skeletal muscle phosphorylase kinase with glycogen was studied by the turbidimetric method at pH 6.8 and 8.2. Binding of phosphorylase kinase by glycogen occurs only in the presence of Ca2+ and Mg2+. The initial rate of complex formation is proportional to the enzyme and polysaccharide concentration; this suggests the formation of a complex with 1:1 stoichiometry in the initial step of phosphorylase kinase binding by glycogen. The kinetic data suggest that phosphorylase kinase substrate--glycogen phosphorylase b--favors the binding of phosphorylase kinase with glycogen. This conclusion is supported by direct experiments on the influence of phosphorylase b on the interaction of phosphorylase kinase with glycogen using analytical sedimentation analysis. The kinetic curves of the formation of the complex of phosphorylase kinase with glycogen obtained in the presence of ATP are characterized by a lag period. Preincubation of phosphorylase kinase with ATP in the presence of Ca2+ and Mg2+ causes the complete disappearance of the lag period. On changing the pH from 6.8 to 8.2, the rate of phosphorylase kinase binding by glycogen is appreciably increased, and complex formation becomes possible even in the absence of Mg2+. A model of phosphorylase kinase and phosphorylase b adsorption on the surface of the glycogen particle explaining the increase in the strength of phosphorylase kinase binding with glycogen in the presence of phosphorylase b is proposed.
KEY WORDS:phosphorylase kinase, glycogen, turbidimetric method, glycogen phosphorylase, analytical ultracentrifugation
Phosphorylase kinase (EC 2.7.1.38) plays a key role in the cascade system of regulation of glycogen metabolism and catalyzes Ca2+-cAMP-dependent phosphorylation and activation of glycogen phosphorylase b [1-3]. The enzyme molecule is a hexadecamer with the subunit formula (alphabetagammadelta)4 and molecular mass 1320 kD [4]. Electron microscopic studies of rabbit skeletal muscle phosphorylase kinase showed that the tight complex of the catalytic gamma-subunits (44.7 kD) with regulatory delta-subunits (16.7 kD) identical to the Ca2+-binding protein calmodulin forms the compact nucleus of the structure, whereas the regulatory alpha (138.4 kD) and beta (125.2 kD) subunits form ridges on the surface of the molecule, giving it the shape of a “butterfly” or “chalice” [5-7].
In skeletal muscle, 20-40% of phosphorylase kinase is localized together with other enzymes of glycogen metabolism on the surface of glycogen granules [8]. Glycogen increases the affinity of phosphorylase kinase to its protein substrate (phosphorylase b) 2-3-fold [9, 10]. The ability of phosphorylase kinase to form a complex with glycogen in the presence of physiological concentrations of Ca2+ and Mg2+ was studied by Steiner and Marshall [11] using sedimentation and turbidimetric methods. The sensitivity of the enzyme for Ca2+ is provided by the delta-subunit [4], whereas both binding sites for Mg2+ are localized in the N-terminal domain of the gamma-subunit [12, 13]. Fisher and Krebs [14] and also Zemskova et al. [10] showed the possibility of a direct influence of glycogen on the rate of autoactivation of phosphorylase kinase by phosphorylation of its alpha- and beta-subunits. The data of Chen and Graves on the activation of the alphagammadelta-complex but not gammadelta-complex under the influence of glycogen suggested the most important role for the alpha-subunit of phosphorylase kinase in the interaction of this enzyme with glycogen [15]. The loss of sensitivity to the activating effect of glycogen of phosphorylase kinase with destroyed alpha-subunit after partial proteolysis also suggests the possible role of the alpha-subunit in the formation of the complex of phosphorylase kinase with glycogen [16]. The formation of this complex was observed also by gel filtration [10]. Shmelev and Serebrenikova, using the turbidimetric method, showed predominant formation of the ternary complex glycogen--phosphorylase kinase--phosphorylase b under the conditions of phosphorylase activity assay, while the formation of a phosphorylase kinase--glycogen complex in the presence of ATP was not detected [17].
The goal of the present paper was to obtain additional information about the mechanism of phosphorylase kinase--glycogen complex formation using the turbidimetric method for continuous monitoring of the kinetics of the complex formation and to study the role of phosphorylase b and ATP in this process in the presence of Ca2+ and Mg2+. Since the non-activated form of phosphorylase kinase is characterized by a considerable increase in activity (up to 20 times) upon changing the of pH from 6.8 to 8.2 [4], it was of interest to compare the ability of phosphorylase kinase to form the complex with glycogen in the presence of physiologically important effectors at both pH values.
MATERIALS AND METHODS
Phosphorylase kinase was obtained from rabbit skeletal muscle according to Cohen [4] using ion-exchange chromatography on DEAE-Toyopearl at the final step of purification [18]. Rabbit skeletal muscle phosphorylase b was prepared by the method of Fisher and Krebs [14] using dithiothreitol instead of cysteine and was recrystallized at least three times. The enzyme was freed of AMP by treatment with Norit A. The preparation of phosphorylase b used here did not contain any phosphorylase a. The concentrations of phosphorylase kinase and phosphorylase b were determined spectrophotometrically using extinction coefficients of 12.4 and 13.2, respectively, for 1% solutions at 280 nm.
Hepes and ATP were purchased from Sigma Chemical Co. (USA). Glycogen from pig liver with average molecular mass 5.5·106 daltons was from Biolar (Latvia). Others reagents (of high purity) were from Russian suppliers.
The kinetics of the formation of the phosphorylase kinase--glycogen complex were followed by the increase in the optical absorbance at 360 nm using a Hitachi 557 spectrophotometer (Japan) equipped with a thermostatted cell holder (1 cm optical path length). The kinetics of the complex formation were studied at 20°C in 40 mM Hepes containing 1 mM beta-mercaptoethanol at pH 6.8 and 8.2.
Sedimentation was studied in a Beckman Spinco model E analytical ultracentrifuge (Austria) equipped with a photoelectric scanner, a multiplexer, and a monochromator. Six-channel rotor An-G-Ti and double-sector cells were used in the experiments. Sedimentation was monitored by measuring the absorbance of the enzyme at 280 nm.
To study the possibility of the existence of a fast stage of interaction between phosphorylase kinase and glycogen, a Durrum D-115 spectrophotometer for rapid kinetics (USA) equipped with a Kel-F cuvette for the monitoring of light scattering was used. The dead time of this spectrophotometer is 20 msec.
RESULTS
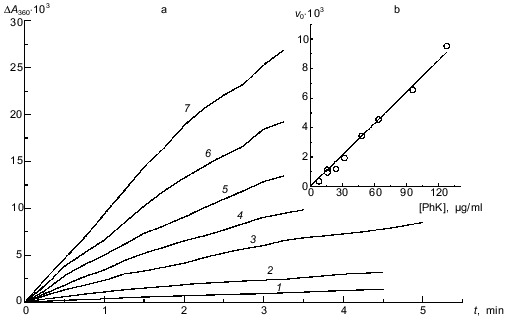
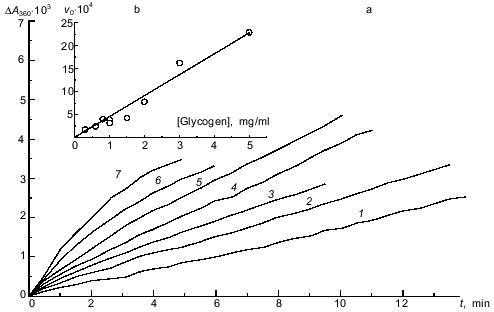
Kinetics of the binding of phosphorylase kinase with glycogen at pH 6.8. Since each glycogen particle of the preparation we used (with molecular mass 5.5·106 daltons) is rather large, it could probably bind several molecules of phosphorylase kinase. Also, since phosphorylase kinase contains specific sites for glycogen binding on its alpha-subunits [15], it may be able to interact with several glycogen particles. To determine the stoichiometry of the phosphorylase kinase--glycogen complex at the initial stage of its formation, we studied the initial rates of the interaction of the enzyme with the polysaccharide at different concentrations of the two components. The kinetics of binding were followed by the increase in the optical absorbance at 360 nm. Figure 1a shows the kinetic curves for complex formation obtained at fixed concentration of glycogen (2 mg/ml) and various concentrations of phosphorylase kinase. The kinetic curves presented in Fig. 2a were obtained at a fixed concentration of phosphorylase kinase (33 µg/ml) and various concentrations of glycogen. As shown in Figs. 1b and 2b, the initial rate of the interaction of phosphorylase kinase with glycogen is directly proportional to the concentration of each component. Thus, we suppose that the stoichiometry of the phosphorylase kinase--glycogen complex at the initial stage of their interaction is 1:1.
Fig. 1. Kinetics of the interaction of phosphorylase kinase (PhK) with glycogen at different enzyme concentrations in 40 mM Hepes, pH 6.8 (20°C). a) Kinetic curves of the change in the optical absorbance at 360 nm (DeltaA360) obtained at different concentrations of phosphorylase kinase (µg/ml): 8 (1), 16 (2), 32 (3), 48 (4), 64 (5), 96 (6), and 128 (7). Complex formation was initiated by the addition of phosphorylase kinase preincubated with Ca2+ and Mg2+ for 2 min at 20°C to the solution containing glycogen (2 mg/ml), 0.1 mM CaCl2, and 10 mM MgCl2. b) Dependence of the initial rate of complex formation v0 (units of optical absorbance per minute) on phosphorylase kinase concentration.
The kinetic curves presented in Figs. 1a and 2a were monitored with a Hitachi-557 spectrophotometer. The monitoring of the curves began approximately 7 sec after addition of phosphorylase kinase. It was of interest to determine if there are fast stages of the interaction of phosphorylase kinase with glycogen in the millisecond interval, as occurs when muscle glycogen phosphorylase b binds with glycogen [19]. Experiments on the mixing of phosphorylase kinase solution with glycogen in the Durrum D-115 spectrophotometer for rapid kinetics showed that in the time interval between 20 msec and 1 sec no noticeable increase in the light scattering intensity occurs. These data suggest that the binding of phosphorylase kinase with glycogen is a relatively slow process. The main changes in the turbidity of the solution occur on the time scale of minutes.Fig. 2. Kinetics of the interaction of phosphorylase kinase and glycogen at various concentrations of the polysaccharide (pH 6.8). a) Kinetic curves of the change in optical absorbance at 360 nm (DeltaA360) obtained at various concentrations of glycogen (mg/ml): 0.3 (1), 0.6 (2), 1.0 (3), 1.5 (4), 2.0 (5), 3.0 (6), and 5.0 (7). Phosphorylase kinase preincubated with Ca2+ and Mg2+ for 2 min at 20°C was added to the solution containing glycogen, 0.1 mM CaCl2, and 10 mM MgCl2. The final concentration of phosphorylase kinase was 33 µg/ml. b) Dependence of the initial rate of complex formation v0 (units of optical absorbance per minute) on the concentration of glycogen.
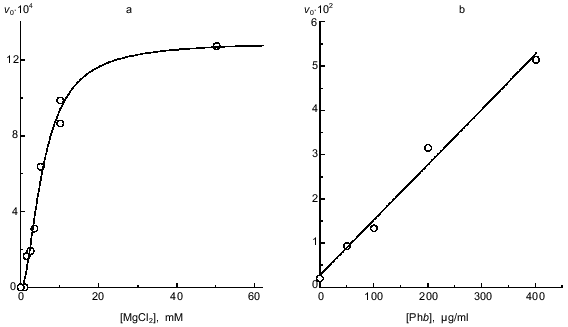
Influence of Mg2+ on complex formation. As shown previously [11, 17], the formation of the phosphorylase kinase--glycogen complex at pH 7.0 occurs only in the presence of Ca2+ and Mg2+. Our research on the role of Ca2+ and Mg2+ supports this conclusion. Figure 3a shows the dependence of the initial rate of the formation of the phosphorylase kinase--glycogen complex (v0) on MgCl2 concentration. The complex does not form in solution without MgCl2. Moreover, we do not observe an increase in the optical absorbance at 1 mM MgCl2 concentration. Thus, the dependence of v0 on MgCl2 concentration is sigmoid. Analysis of this dependence using the Hill equation [20] gives a Hill coefficient of 1.8 ± 0.2; at 5.8 ± 0.6 mM Mg2+, v0 is equal to half of the value obtained at saturating MgCl2 concentration. It is interesting to note that Steiner and Marshall [11] observed the sigmoid dependence of the change of optical density at 350 nm on Mg2+ concentration if it was measured within 3 h after the mixing of phosphorylase kinase and glycogen.
Influence of phosphorylase b on complex formation. A large increase in the initial rate of phosphorylase kinase binding with glycogen is observed in the presence of the substrate of kinase reaction--phosphorylase b. The dependence of v0 on phosphorylase b concentration remains linear up to phosphorylase b concentration 400 µg/ml (Fig. 3b). These data indicate that phosphorylase b favors the binding of phosphorylase kinase with glycogen.Fig. 3. Influence of Mg2+ and phosphorylase b (Phb) on the kinetics of the interaction of phosphorylase kinase with glycogen (pH 6.8). a) Dependence of the initial rate of complex formation v0 (units of optical absorbance per minute) on MgCl2 concentration. Phosphorylase kinase preincubated with Ca2+ and Mg2+ was added to the solution containing glycogen (1 mg/ml), 0.1 mM CaCl2, and various concentrations of MgCl2. The final concentration of phosphorylase kinase was 70 µg/ml. b) Dependence of v0 on phosphorylase b concentration. Phosphorylase kinase preincubated with Ca2+ and Mg2+ was added to the solution containing glycogen (0.6 mg/ml), 0.1 mM CaCl2, 10 mM MgCl2, and various concentrations of phosphorylase b. The final concentration of phosphorylase kinase was 35 µg/ml. Complex formation was initiated by the addition of phosphorylase kinase preincubated with Ca2+ and Mg2+ for 2 min at 20°C to the solution containing glycogen (a) or glycogen and phosphorylase b (b).
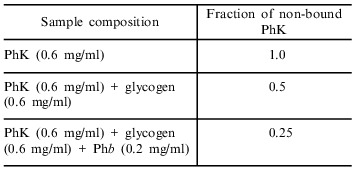
Direct support of an increase in the strength of phosphorylase kinase binding with glycogen in the presence of phosphorylase b was obtained by sedimentation analysis. Phosphorylase kinase (0.26 mg/ml) sediments at pH 6.8 and 20°C with sedimentation coefficient 21S. When phosphorylase kinase is sedimented in the presence of glycogen (0.6 mg/ml), a decrease in the height of the boundary corresponding to the sedimentation of the free enzyme by twofold occurs (the table). This decrease in the height of the boundary corresponds to a decrease in the concentration of the free phosphorylase kinase as a result of its binding with glycogen. When phosphorylase kinase is sedimented in the presence of glycogen and phosphorylase b (0.2 mg/ml) a further twofold decrease in the height of the boundary of sedimentation corresponding to free phosphorylase kinase occurs. Thus, the presence of phosphorylase b in the system causes tighter binding of phosphorylase kinase by glycogen. This is probably explained by an interaction of phosphorylase kinase and phosphorylase b on the surface of the glycogen particle which causes tighter binding of phosphorylase kinase with glycogen. This idea was first proposed by Serebrenikova and Shmelev [21].
Influence of phosphorylase b (Phb) on the binding of phosphorylase kinase (PhK) with glycogen according data of sedimentation analysis. Composition of buffer: 40 mM Hepes, 0.1 mM Ca2+, 10 mM Mg2+, pH 6.8, 20°C
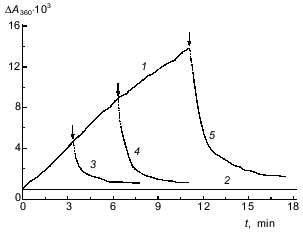
Reversibility of complex formation. If the formation of the phosphorylase kinase--glycogen complex is reversible, the binding by EGTA of Ca2+ (which is absolutely necessary for complex formation) should cause the dissociation of the complex. In fact, as shown in Fig. 4, the addition of 1 mM EGTA to the system where the complex formation is monitored causes a distinct decrease in the optical absorption of the solution; this is probably associated with the dissociation of the phosphorylase kinase--glycogen complex (curves 3-5 in Fig. 4). Our analysis of the kinetic curves of the dissociation of the complex showed that they followed the exponential law with rate constant 1.5 ± 0.2 min-1. These results suggest that the binding of phosphorylase kinase with glycogen is reversible.
Influence of ATP on complex formation. Shmelev and Serebrenikova [17] showed that 3 mM ATP decreases the binding of phosphorylase kinase with glycogen. They followed the process of complex formation for 4 min. We studied the influence of ATP on complex formation in detail. We found that in the presence of ATP a lag period appears on the curves of the time dependence of the increase in the optical absorbance (Fig. 5a). The duration of the lag period (tau) was calculated from the segment which is crossed on the abscissa by the extension of the steady-state part of the kinetic curve. The value tau increases when ATP concentration increases (Fig. 5b). It should be noted that when ATP concentration is increase the stationary rate of complex formation (vst) decreases, and a noticeable decrease in vst (1.8-fold) occurs at 0.015 mM ATP concentration.Fig. 4. Dissociation of the phosphorylase kinase--glycogen complex by EGTA (pH 6.8). The kinetic curves of the interaction of phosphorylase kinase (50 µg/ml) with glycogen (2 mg/ml) in the presence of 0.1 mM CaCl2, 10 mM MgCl2 (1) or 0.1 mM CaCl2, 10 mM MgCl2, 1 mM EGTA (2). Curves 3-5 correspond to the dissociation of the complex after the addition of EGTA (final concentration 1 mM). The points of EGTA addition are indicated by the arrows.
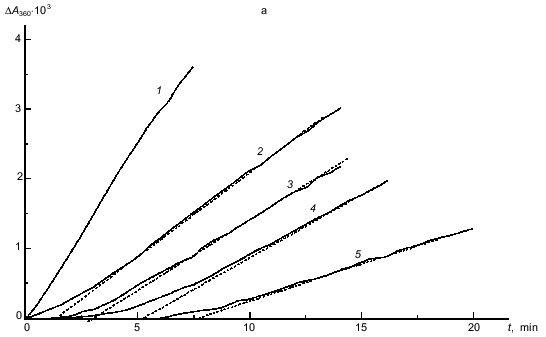
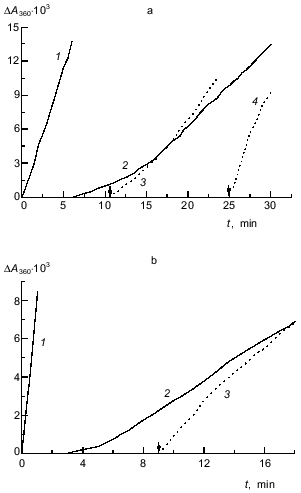
In the experiments shown in Fig. 5a, phosphorylase kinase was added to the solution containing glycogen and ATP. It was interesting to study the influence of preincubation of phosphorylase kinase with ATP (in the presence of Mg2+ and Ca2+) on the kinetics of the binding of the enzyme with glycogen. ATP concentration in these experiments was 0.15 mM. The kinetic curve obtained on addition of the mixture of glycogen and ATP to a solution containing phosphorylase kinase, 0.1 mM CaCl2, and 10 mM MgCl2 (curve 2 in Fig. 6a) served as a control. The duration of the lag period for this curve was 11.5 min. If phosphorylase kinase was preincubated with 0.15 mM ATP for 11 min (this time is not enough to reach the steady-state part on curve 2 in Fig. 6a) and then complex formation was initiated by addition of glycogen, the lag period on the kinetic curve was preserved (curve 3; tau ~ 2.5 min). In this case a pronounced increase in vst (1.4-fold) was observed as compared to the value of vst for curve 2. When phosphorylase kinase was preincubated with 0.15 mM ATP for 25 min (this time interval corresponds to the steady-state part for curve 2), the lag period was absent (curve 4) and the steady-state (in this case initial) rate coincided with the initial rate of complex formation in the absence of ATP (curve 1; v0 = 2.3 units of optical absorbance per minute).Fig. 5. Influence of ATP on the kinetics of the interaction of phosphorylase kinase with glycogen (pH 6.8). a) Kinetic curves of the change in optical absorbance at 360 nm (DeltaA360) obtained at various concentrations of ATP (mM): 0 (1), 0.015 (2), 0.06 (3), 0.15 (4), and 1.5 (5). Phosphorylase kinase preincubated with Ca2+ and Mg2+ was added to the solution containing glycogen (0.7 mg/ml), 0.1 mM CaCl2, 10 mM MgCl2, and various concentrations of ATP. The final concentration of phosphorylase kinase was 80 µg/ml. b) Dependence of the duration of the lag period on ATP concentration. c) Dependence of the stationary rate of the interaction of phosphorylase kinase with glycogen vst (units of optical absorbance per minute) on the concentration of ATP.
As shown in [17], phosphorylase b removes the inhibitory effect of ATP, allowing a rather high rate for the interaction of phosphorylase kinase with glycogen. It is apparent that the effect of phosphorylase b should be studied keeping in mind the fact that a stepwise increase in complex formation rate occurs in the presence of ATP (“acceleration” on the kinetic curves). Kinetic curve 2 in Fig. 6b was obtained when a mixture of glycogen and ATP was added to a solution containing phosphorylase kinase and phosphorylase b (final concentration 0.15 mM ATP). These conditions are different from those for curve 2 in Fig. 6a by the presence of phosphorylase b. The duration of the lag period for the kinetic curve obtained in the presence of phosphorylase b and ATP (tau ~ 5 min) is distinctly lower than the value of tau for the kinetic curve obtained in the presence of only ATP (11.5 min). The lag period on the kinetic curve is absent if phosphorylase kinase is preincubated with phosphorylase b and ATP for a time interval which is enough to reach the steady-state part of kinetic curve 2 in Fig. 6b (curve 3, preincubation time 9 min). The initial rate of complex formation for this curve remains markedly less than the initial rate in the absence of ATP (curve 1 in Fig. 6b). Thus, the addition of phosphorylase b to the system containing phosphorylase kinase, glycogen, and ATP causes more rapid attainment of the steady-state part. This result is in a good agreement both with our data that phosphorylase b favors the binding of phosphorylase kinase with glycogen and with the results of Shmelev and Serebrenikova showing the binding of phosphorylase kinase with glycogen on addition of phosphorylase b to a solution containing phosphorylase kinase, glycogen, ATP, Ca2+, and Mg2+.Fig. 6. Influence of the preincubation of phosphorylase kinase with ATP on the kinetics of the interaction of the enzyme with glycogen in the absence (a) and in the presence (b) of phosphorylase b. a) Curves of the changes in optical absorbance at 360 nm (DeltaA360) obtained on addition of phosphorylase kinase preincubated with Ca2+ and Mg2+ to the solution containing glycogen, 0.1 mM CaCl2, and 10 mM MgCl2; the final concentration of ATP, 0.15 mM (1); on addition of the mixture of glycogen and ATP to the solution containing phosphorylase kinase, 0.1 mM CaCl2, and 10 mM MgCl2 (2); on addition of glycogen to phosphorylase kinase preincubated with 0.15 mM ATP in the presence of 0.1 mM CaCl2 and 10 mM MgCl2 for 11 (3) or 25 min (4). b) Curves of the changes in optical absorbance at 360 nm (DeltaA360) obtained on addition of phosphorylase kinase preincubated with Ca2+ and Mg2+ to the solution containing glycogen, phosphorylase b, 0.1 mM CaCl2, and 10 mM MgCl2 (1); on addition of the mixture of glycogen and ATP to the solution containing phosphorylase kinase, phosphorylase b, 0.1 mM CaCl2, and 10 mM MgCl2; final concentration of ATP, 0.15 mM (2); on addition of glycogen to phosphorylase kinase preincubated with phosphorylase b, 0.15 mM ATP, 0.1 mM CaCl2, and 10 mM MgCl2 for 9 min (3). Final concentrations: phosphorylase kinase, 48 µg/ml; glycogen, 0.7 mg/ml; phosphorylase b (for Fig. 6b), 110 µg/ml. The points of glycogen addition are indicated by the arrows.
It should be noted that when the system also contains ATP and phosphorylase b, in addition to phosphorylase kinase, glycogen, Ca2+, and Mg2+, the conditions for the phosphorylase kinase reaction are reached. This means that curves 2 and 3 in Fig. 6b are obtained under conditions when phosphorylase b is transformed into the phosphorylated form, phosphorylase a. Thus, strictly speaking, the effect of phosphorylase under these conditions is the mixed effect of forms a and b.
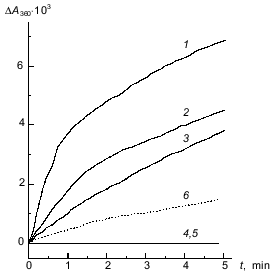
Kinetics of binding of phosphorylase kinase with glycogen at pH 8.2. Typical curves for binding of phosphorylase kinase with glycogen at pH 8.2 are presented in Fig. 7 (curves 1-5). Curve 1 was obtained at phosphorylase kinase and glycogen concentrations of 80 µg/ml and 0.6 mg/ml, respectively. The rate of complex formation at pH 6.8 under the same conditions is substantially lower (curve 6). The rate of this process occurs is also less when Mg2+ is absent (curve 2) or ATP is added (curve 3). In the presence of EGTA, which binds Ca2+, phosphorylase kinase does not interact with glycogen (curves 4 and 5). Thus, the significant difference in the process of complex formation at pH 8.2 versus pH 6.8 is the possibility for the enzyme to bind with glycogen in the absence of Mg2+.
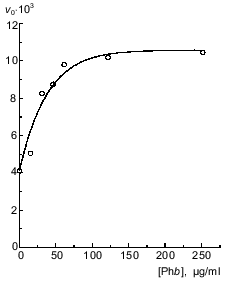
Like the experiments at pH 6.8, at pH 8.2 the rate of phosphorylase kinase binding with glycogen is increased in the presence of phosphorylase b (Fig. 8). But in this case, at phosphorylase b concentration close to 250 µg/ml, the initial rate of complex formation reaches a limiting value.Fig. 7. Interaction of phosphorylase kinase with glycogen in 40 mM Hepes buffer, pH 8.2 (20°C). Kinetic curves of the changes in the optical absorbance at 360 nm (DeltaA360) obtained on addition of phosphorylase kinase to glycogen solution containing: 0.1 mM CaCl2 and 10 mM MgCl2 (1); 0.1 mM CaCl2 (2); 0.1 mM CaCl2, 10 mM MgCl2, and 1.5 mM ATP (3); 1 mM EGTA (4); 1 mM EGTA, 10 mM MgCl2 (5). The dashed line (6) corresponds to the kinetic curve obtained on addition of phosphorylase kinase to a solution containing glycogen, 0.1 mM CaCl2, and 10 mM MgCl2 in 40 mM Hepes buffer, pH 6.8. Final concentrations: phosphorylase kinase, 80 µg/ml; glycogen, 0.6 mg/ml.
Fig. 8. Dependence of the initial rate of the interaction of phosphorylase kinase with glycogen v0 (units of optical absorbance per minute) on the concentration of phosphorylase b (Phb) at pH 8.2. Complex formation was initiated by addition of phosphorylase kinase preincubated with Ca2+ and Mg2+ to a solution containing glycogen (0.6 mg/ml) and phosphorylase b at various concentrations. The final concentration of phosphorylase kinase was 80 µg/ml.
DISCUSSION
One of the peculiarities of the process of interaction of phosphorylase kinase with glycogen at pH 6.8 is the fact that in the presence of a rather high concentration of ATP (1.5 mM) the initial rate of complex formation is zero. But in some time the rate of the process substantially increases. Moreover, after preincubation of phosphorylase kinase with ATP, Ca2+, and Mg2+, the ability of the enzyme to interact with glycogen is completely recovered (the initial rate of complex formation reaches a value corresponding to that in the absence of ATP).
It is known that preincubation of phosphorylase kinase with ATP, Ca2+, and Mg2+ causes the autophosphorylation of the enzyme on the alpha- and beta-subunits [22, 23]. Usually, in experiments on autophosphorylation of phosphorylase kinase, 3 mM ATP, 0.1 mM CaCl2, and 10 mM MgCl2 are used [22]. The binding of ATP by phosphorylase kinase probably induces some conformational changes in the enzyme molecule which causes the loss of its ability to bind with glycogen. Perhaps after autophosphorylation of phosphorylase kinase the effect of ATP gradually disappears.
If the appearance of the ability of phosphorylase kinase to bind with glycogen after preincubation with ATP, Ca2+, and Mg2+ is really connected with the process of autophosphorylation, then the accelerating effect of phosphorylase b on complex formation under these conditions is probably connected with an increase in the rate of autophosphorylation of phosphorylase kinase in the presence of the protein substrate.
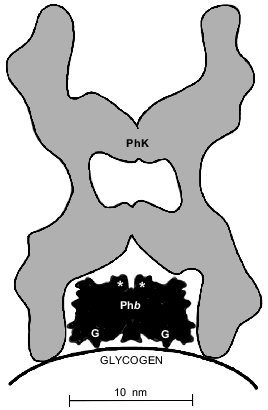
Since phosphorylase b binds with glycogen more quickly than phosphorylase kinase [19], one might expect that phosphorylase b would prevent the adsorption of phosphorylase kinase on the glycogen particle. However, our data suggest that phosphorylase b favors the formation of the complex of phosphorylase kinase with glycogen. A scheme for adsorption of phosphorylase b and phosphorylase kinase on the surface of the glycogen particle proposed by us (Fig. 9) explains how phosphorylase b favors the tighter binding of phosphorylase kinase with glycogen. The molecular dimensions of the dimer of phosphorylase b (6.5 × 6.5 × 11 nm) are taken from the paper of Barford and Johnson [24]. According to the electron microscopic data [7], the phosphorylase kinase molecule more often has a “butterfly” form with dimensions 22.2 × 29 nm. The “butterfly” wings are formed by the alpha- and beta-subunits and are connected with bridges consisting of the gamma- and delta-subunits. In the enzyme--substrate complex formed by phosphorylase kinase and phosphorylase b, the phosphorylase molecule is situated between the “butterfly” wings in such a way that the phosphorylating residues of Ser-14 are turned to the catalytic gamma-subunits of phosphorylase kinase. Such orientation of phosphorylase b in the enzyme--substrate complex was first suggested in [5].
According to X-ray data [24], glycogen-binding sites in dimeric phosphorylase b (glycogen storage sites) are situated on the opposite side relative to the phosphorylating Ser-14 residues. Keeping in mind that the glycogen-binding sites of phosphorylase kinase are situated on its alpha-subunits [15], it is completely reasonable to propose a structure for the ternary glycogen--phosphorylase b--phosphorylase kinase complex where phosphorylase b is enclosed in a "cave" formed by phosphorylase kinase and the surface of the glycogen particle. It is important to note that the formation of such a complex is strongly ordered: first, on the surface of the glycogen particle, phosphorylase b dimer is bound; then the adsorbed phosphorylase molecule is “covered” by the phosphorylase kinase molecule. In such a complex the phosphorylase kinase is held on the surface of the glycogen particle not only by its direct contacts with glycogen, but also by contacts with the adsorbed phosphorylase b. This pattern could explain the tighter binding of phosphorylase kinase with glycogen in the presence of phosphorylase b. In the case when the system contains ATP, the ordered binding of phosphorylase kinase and phosphorylase b on the surface of the glycogen particle is only possible, we suppose, after autophosphorylation of phosphorylase kinase. Because in vivo autophosphorylation is unlikely [25], we suppose that in the cell the formation of such an ordered ternary complex proceeds after phosphorylation of phosphorylase kinase by a cAMP-dependent protein kinase in response to a hormonal signal.Fig. 9. Scheme for the binding of phosphorylase kinase (PhK) and phosphorylase b (Phb) on a glycogen particle. G are glycogen storage sites in the dimeric molecule of phosphorylase b; the location of the Ser-14 residues which can be phosphorylated is indicated by the asterisks.
The authors are very thankful to S. V. Klinov for help in carrying out experiments with the D-115 spectrophotometer for rapid kinetics.
This work was financially supported by the Russian Foundation for Basic Research (grants 96-04-50819 and 96-04-49243).
REFERENCES
1.Krebs, E. G., Graves, D. J., and Fisher, E. H.
(1959) J. Biol. Chem., 234, 2867-2873.
2.Hayakawa, T., Perkins, J. P., Walsh, D. A., and
Krebs, E. G. (1973) Biochemistry, 12, 567-573.
3.Livanova, N. B. (1993) Biochemistry
(Moscow), 58, 1234-1239 (Russ.).
4.Cohen, P. (1973) Eur. J. Biochem.,
34, 1-14.
5.Schramm, H. J., and Jennissen, H. P. (1985) J.
Mol. Biol., 181, 503-516.
6.Wilkinson, D. A., Marion, T. N., Tillman, D. M.,
Norcum, M. T., Hainfeld, J. F., Seyer, J. M., and Carlson, G. M. (1994)
J. Mol. Biol., 235, 974-982.
7.Norcum, M. T., Wilkinson, D. A., Carlson, M. Ch.,
Hainfeld, J. F., and Carlson, G. M. (1994) J. Mol. Biol.,
241, 94-102.
8.Meyer, E., Heilmeyer, L. M. G., Hashke, R. H., and
Fisher, E. H. (1970) J. Biol. Chem., 245, 6642-6648.
9.Morange, M., and Buc, H. (1979) Biochimie,
61, 633-643.
10.Zemskova, I. A., Shur, S. A., Skolysheva, L.
K., and Vulfson, P. L. (1989) Biokhimiya, 54,
662-668.
11.Steiner, R. F., and Marshall, L. (1982)
Biochim. Biophys. Acta, 707, 38-45.
12.Huang, C.-Y. F., Yuan, C.-J., Luo, S., and
Graves, D. J. (1994) Biochemistry, 33, 5877-5883.
13.Huang, C.-Y. F., Yuan, C.-J., Livanova, N. B.,
and Graves, D. J. (1993) Mol. Cell. Biochem., 127/128,
7-18.
14.Fisher, E. H., and Krebs, E. G. (1962) Meth.
Enzymol., 5, 369-374.
15.Chan, K. F., and Graves, D. J. (1982) J. Mol.
Biol., 257, 5939-5947.
16.Shur, S. A., Pegova, A. I., Skolysheva, L.
K., and Vulfson, P. L. (1982) Biokhimiya, 47,
266-271.
17.Shmelev, V. K., and Serebrenikova, T. P. (1997)
Biochem. Mol. Biol. Int., 43, 867-872.
18.Morozov, V. E., Eronina, O. B., Andreeva, I.
E., Silonova, G. V., Solovyeva, N. V., Shchors, E. I., Livanova, N. B.,
and Poglazov, B. F. (1989) Biokhimiya, 54, 448-455.
19.Klinov, S. V., Davydov, D. R., Lisovskaya, N. P.,
and Kurganov, B. I. (1980) Mol. Biol. (Moscow), 14,
348-356.
20.Kurganov, B. I. (1968) Allosteric Enzymes
[in Russian], Nauka, Moscow, p. 28.
21.Serebrenikova, T. P., and Shmelev, V. K. (1989)
in Abst. Int. Symp. Molecular Organization of Biological
Structures, Moscow, Vol. 1, p. 131.
22.Wang, J. H., Stull, J. T., Huang, T.-S., and
Krebs, E. G. (1976) J. Biol. Chem., 251, 4521-4527.
23.Carlson, G. M., and Graves, D. J. (1976) J.
Biol. Chem., 251, 7480-7486.
24.Barford, D., and Johnson, L. N. (1989)
Nature, 340, 609-616.
25.Cohen, P. (1974) Biochem. Soc. Symp.,
39, 51-73.