Determination of Disulfide Bonds in gamma-46 Gliadin
T. A. Egorov1*, T. I. Odintsova2, and A. K. Musolyamov1
1Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, ul. Vavilova 32, Moscow, 117984 Russia; fax: (095) 135-1405; E-mail: egorov@imb.imb.ac.ru2Vavilov Institute of General Genetics, Russian Academy of Sciences, ul. Gubkina 3, Moscow, 117809 Russia
* To whom correspondence should be addressed.
Received July 3, 1998; Revision received December 3, 1998
The disulfide bonds in gamma-46 gliadin were identified: Cys173--Cys192, Cys212--Cys291, Cys165--Cys199 (or Cys200), Cys283--Cys200 (or Cys199). The disulfide-containing peptides were obtained by limited hydrolysis of the intact protein with chymotrypsin at an enzyme/substrate ratio of 1:1000 at 20°C for 22 h with subsequent digestion of disulfide-containing fragments with trypsin and chymotrypsin. The locations of disulfide bonds were determined by sequencing disulfide-containing fractions and constituent peptides and comparison of the obtained sequences with the partial amino acid sequence of gamma-46 gliadin determined earlier.
KEY WORDS: storage proteins, prolamins, gliadins, disulfide bonds
The main storage proteins of wheat grains, also known as gluten proteins, are classically subdivided into the monomeric gliadins, which either contain (alpha- and gamma-type gliadins) or lack cysteine residues (omega-type gliadins), and the polymeric glutenins consisting of high- and low-molecular-weight subunits. The major difference between these two groups of proteins consists in the ability of high- and low-molecular-weight glutenin subunits to form polymers stabilized by inter-chain disulfide bonds, while the gliadins are monomeric proteins with intra-chain disulfide bonds [1]. Disulfide bonds play a key role in the formation and viscoelastic properties of the gluten. However, the determination of disulfide bonds in prolamins is a challenging problem due to the unusual sequences and properties of these proteins and their high heterogeneity. Nevertheless, in recent years considerable progress has been achieved in this field [2]. However, it should be noted that the disulfide bonds were determined in alpha- and gamma-type gliadins of unknown structure, and the proteins isolated were not characterized except for the number of cysteine residues [3, 4]. The determination of disulfide bonds in these works was based on several known amino acid sequences of the alpha- and gamma-type gliadins [5-9], which are highly homologous, especially around the cysteine residues. It is evident that these results provide a general idea of the disulfide bond arrangement in the gluten proteins; however, it is impossible to assign these data to a particular protein, taking into account that about 50 proteins are present in wheat endosperm. Therefore, these data need to be confirmed. Earlier, in order to identify disulfide bonds in gliadins, we determined the partial amino acid sequence of gamma-46 gliadin and showed by mass spectrometry that all eight cysteine residues of this protein were involved in intra-chain disulfide bonds [10].
The objective of this work was to locate the disulfide bonds in gamma-46 gliadin. Preliminary results on this subject were reported earlier [11].
MATERIALS AND METHODS
Materials. Trypsin treated with tosylphenylalanine chloromethyl ketone, Tris-HCl, and guanidine hydrochloride were obtained from Sigma (USA). Chymotrypsin (3× recrystallized) was from Serva (Germany). 4-Vinylpyridine (Fluka, Switzerland) was distilled under argon. All reagents and solvents for sequencing were obtained from Applied Biosystems (USA). Acetonitrile UV was obtained from Lekbiofarm (Russia). Water was purified using a Milli Q System (Millipore, USA). All other reagents were of analytical grade.
Protein purification. The gamma-46 gliadin isolated from the Hardi cultivar was provided by Y. Popineau (Laboratory of Technology and Biochemistry of Proteins, Nantes, France). It was additionally purified by RP-HPLC on an Aquapore RP-300 column (4.6 x 220 mm) with a linear acetonitrile gradient from 20 to 50% for 40 min at a flow rate of 0.5 ml/min. Solvent A was 0.1% (v/v) TFA in water, and solvent B was acetonitrile in 0.08% (v/v) TFA.
Limited hydrolysis of gamma-46 gliadin with chymotrypsin. One milligram of the intact protein was digested with 1 µg of chymotrypsin in 1 ml of 0.05 M ammonium bicarbonate buffer containing 10% dioxane and 0.002 M CaCl2 at 22°C for 22 h. The peptides obtained were separated by RP-HPLC. This allowed us to isolate a disulfide-containing fragment (fraction 10) [10].
Exhaustive enzymatic hydrolysis of disulfide-containing fragments. Fraction 10 obtained by limited hydrolysis of the intact gamma-46 gliadin with chymotrypsin was further digested with 5 µg of trypsin at 37°C for 6 h. The disulfide-containing fraction 10-5 was further digested with 5 µg of chymotrypsin at 37°C for 16 h. The peptides were separated by RP-HPLC.
Identification of disulfide-containing fragments. The disulfide-bonded peptides isolated by RP-HPLC were identified, as described in [10], by reduction and alkylation of the chromatographic fractions with subsequent separation of peptides in the same acetonitrile gradient as for separation of the original mixture.
Determination of N-terminal amino acid sequences was performed on a model 816 protein sequencer (Knauer, Germany) supplied with a PTH Analyzer (Applied Biosystems, USA) operating according to the manufacturers' protocols. Peptide samples were dissolved in 30% acetonitrile containing 0.1% TFA and applied to Immobilon P membrane (Millipore, USA) pretreated with polybrene (Sigma) and subjected to three cycles of sequencing.
RESULTS AND DISCUSSION
Several complete amino acid sequences of alpha- and gamma-type gliadins deduced from cDNA are presently available [5-9]. alpha- and gamma-type gliadins differ not only in size, but in the number of cysteine residues as well: the alpha-type gliadins contain six cysteine residues, while gamma-gliadins have eight cysteine residues, and six of them are conserved. The sequences around cysteine residues are highly homologous [2]. Using modern methods of protein chemistry, we determined the partial amino acid sequence (207 amino acid residues) of gamma-46 gliadin [10]. Using time-of-flight electrospray mass spectrometry, we were the first to show that this protein contains eight cysteine residues, and all of them are involved in the formation of disulfide bonds. In addition, by limited hydrolysis of intact gamma-46 gliadin with chymotrypsin, we isolated a disulfide-containing fragment (fraction 10). The primary structure of gamma-46 gliadin was most similar to the pW1621 clone gliadin (308 amino acid residues) [8], whose structure was later refined: Ser-173 was substituted by Cys173 (G. Gallili, personal communication). The Mr (measured) of gamma-46 gliadin is 35191.3, while Mr (calculated without considering disulfide bonds) of the clone pW1621 gliadin is 35144.6. In both proteins the number of cysteine residues and surrounding amino acid sequences are similar. Of 207 amino acid residues determined in gamma-46, only three substitutions (compared to the pW1621 clone gliadin) were found [10]. So, for convenience, the amino acid residues in the gamma-46 gliadin were numbered as in the clone pW1621 gliadin [8].
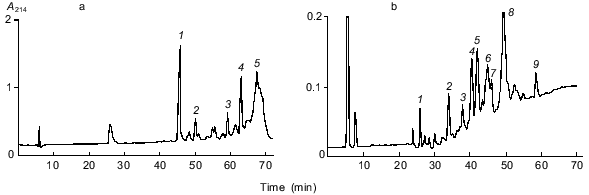
In this work, to locate disulfide bonds in gamma-46, the disulfide-containing fraction 10 was first digested with trypsin, and the peptides obtained were separated by RP-HPLC (the figure, panel (a)). For identification of disulfide-containing fragments, the main chromatographic fractions were reduced, alkylated with 4-vinylpyridine, and separated by RP-HPLC under the same conditions as for fraction 10. The peptides obtained and the original disulfide-containing fractions were sequenced. Accordingly, we identified two major disulfide-containing fractions: fraction 10-4 and fraction 10-5. Fraction 10-4 consisted of two peptides containing Cys173 (K) and Cys192 (L) (designations of cysteine residues in the superfamily of cereal prolamins are given in [12]), and fraction 10-5 consisted of three peptides containing six cysteine residues Cys165 (B), Cys199 (C), Cys200 (D), Cys212 (F), Cys283 (G), and Cys291 (H) (the table). Fraction 10-5 was further subjected to exhaustive hydrolysis with chymotrypsin, and the products of the reaction were separated by RP-HPLC (the figure, panel (b)). The subsequent analysis allowed us to identify two cysteine-containing fractions: 10-5-5 and 10-5-8 (the table). Of them, fraction 10-5-5 consisted only of two peptides containing cysteine residues Cys212 (F) and Cys291 (H), while fraction 10-5-8 consisted of three peptides containing Cys165 (B), Cys199 (C), Cys200 (D), and Cys283 (G). As follows from the partial structure of gamma-46, two cysteine residues, Cys199 (C) and Cys200 (D), are adjacent. These results show that Cys165 (B) is either connected with Cys199 (C) or with Cys200 (D); and similarly, Cys283 (G) is either linked to Cys199 (C) or Cys200 (D). All attempts to identify disulfide bonds involving adjacent cysteine residues were unsuccessful. Recently, the disulfide bonds in gamma- [4] and alpha-type [3] gliadins of unknown structure and in gamma-46 gliadin (H. Wieser, personal communication) have been reported. These data agree with our findings. The arrangement of disulfide bonds in these proteins was determined by sequencing disulfide-containing fragments and comparison of the obtained sequences with alpha- and gamma-gliadins, whose amino acid sequences were deduced from the cDNA of hexaploid wheat. In these communications, as in our work, the disulfide bonds involving adjacent cysteine residues Cys199 (C) and Cys200 (D) were not located. Our data show that gamma-gliadins have four disulfide bridges (K--L, B--C, or D, G--B or C, and F--H) and lack free sulfhydryl groups. Comparative analysis indicates that, in alpha-type gliadins, the disulfide bridge F--H is missing.
Disulfide-containing fragments of gamma-46 gliadinRP-HPLC separation of disulfide-containing peptides of gamma-46 gliadin on an Aquapore RP-300 column (4.6 x 220 mm). Peptides were eluted with an acetonitrile gradient at a flow rate of 0.5 ml/min. Solvent A, 0.1% TFA; solvent B, acetonitrile in 0.1% TFA. a) Separation of the tryptic peptides of fraction 10. Peptides were eluted with an acetonitrile gradient: 0-40% B for 60 min and 40-60% B for 30 min. b) Separation of the chymotryptic peptides of fraction 10-5 (panel (a)) with an acetonitrile gradient from 0 to 50% B for 70 min.
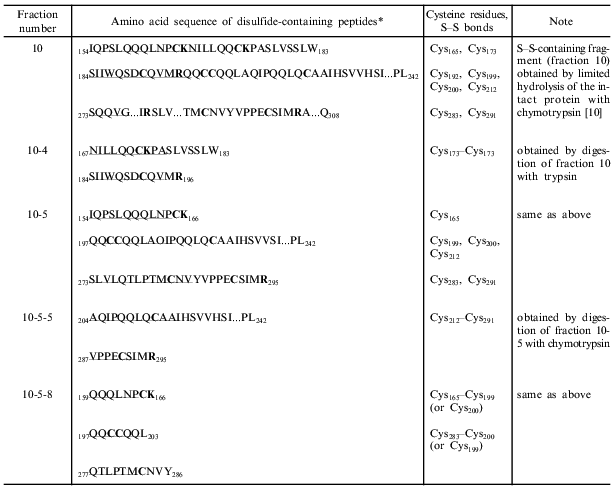
*Sequenced amino acid sequences of disulfide-containing fragments are underlined. The partial amino acid sequence of gamma-46 necessary for the identification of disulfide-containing fragments is shown in [10]. Cysteine, lysine, and arginine residues are given in bold.
This work was supported in part by the Russian Foundation for Basic Research (grant No. 96-04-49850).
REFERENCES
1.Shewry, P. R. (1995) Biol. Rev., 70,
375-426.
2.Shewry, P. R. (1997) J. Cereal Sci.,
25, 207-227.
3.Müller, S., and Wieser, H. (1995) J. Cereal
Sci., 22, 21-27.
4.Müller, S., and Wieser, H. (1997) J. Cereal
Sci., 26, 169-176.
5.Kasarda, D. D., Okita, T. W., Bernardin, J. E.,
Baecker, P. A., Nimmo, C. C., Lew, E. J.-L., Dietler, M. D., and
Greene, F. C. (1984) Proc. Natl. Acad. Sci. USA, 81,
4812-4716.
6.Bartels, D., Altosaar, J., Harberd, N. P., Barker,
R. F., and Thompson, R. D. (1986) Theor. Appl. Genet.,
72, 845-853.
7.Rafalski, J. A. (1986) Gene, 43,
221-229.
8.Sugiyama, T., Rafalski, J. A., and Soll, D. (1986)
Plant Sci., 44, 205-209.
9.Scheets, K., and Hedgcoth, C. (1988) Plant
Sci., 57, 141-150.
10.Egorov, T. A., Odintsova, T. I., Musolyamov, A.
K., Barbashov, S. F., Pustobaev, V. N., Andersen, J., Roepstorff, P.,
and Popineau, Y. (1998) Biochemistry (Moscow), 63, 59-66
(Russ.).
11.Egorov, T. A., Musolyamov, A. K., Barbashov, S.
F., Zolotykh, O. T., Andersen, J., Roepstorff, P., and Popineau, Y.
(1994) in Wheat Kernel Proteins. Molecular and Functional Aspects.
Proc. Int. Meet. (Universita degli Studi della Tuscia, ed.),
Viterbo, Italy, pp. 41-44.
12.Egorov, T. A., Odintsova, T. I., Musolyamov, A.
K., Tatham, A., and Shewry, P. (1996) FEBS Lett., 396,
285-288.