Anionic Carbohydrate-Containing Polymers of Cell Walls in Two Streptoverticille Genospecies
Yu. I. Kozlova1, G. M. Streshinskaya1, A. S. Shashkov2, L. I. Evtushenko3, and I. B. Naumova1*
1School of Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; E-mail: naumova@1.micro.bio.msu.ru2Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky pr. 47, Moscow, 117913 Russia; fax: (095) 135-5328
3Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Pushchino, Moscow Region, 142292 Russia; fax: (095) 923-3602
* To whom correspondence should be addressed.
Received July 29, 1998; Revision received September 20, 1998
The cell walls of two streptoverticille genospecies which belong to a historically isolated group of the genus Streptomyces contain anionic polymers of different structure. Streptomyces hachijoensis VKM Ac-191T and Streptomyces cinnamoneus subsp. azacoluta VKM Ac-606T assigned to one genospecies on the basis of DNA--DNA hybridization [5] contain 37% of an identical sugar-1-phosphate polymer. The repeating disaccharide units of the polymer, 2-amino-2-deoxy-alpha-D-glucopyranosyl-(1-->6)-2-acetamido-2-deoxy-alpha-D-glucopyranose, are linked at C-1 and C-6' by phosphodiester bonds. The cell walls of Streptomyces biverticillatus VKM Ac-891T and Streptomyces baldaccii VKM Ac-821T, members of another genospecies, contain about 30% 1,3-poly(glycerol phosphate) completely substituted by 2-amino-2-deoxy-alpha-D-glucopyranosyl residues at C-2. Due to the presence of an amino sugar with a free amino group in the repeating unit, the polymers exhibit neutral properties. Polymer structures were determined by chemical methods and NMR spectroscopy. The data indicate taxonomic specificity of anionic polymers in streptoverticille cell walls.
KEY WORDS: cell wall, teichoic acid, sugar-1-phosphate polymer, streptoverticille, NMR spectroscopy
Analysis of the chemical composition of prokaryotic cells and cell walls has attracted considerable interest. This is mainly due to the search for new chemotaxonomic genus- and species-specific markers in bacteria, since phenotypic characters are usually ineffective for differentiating numerous new taxa revealed by molecular biological methods at the phylogenetic level.
Anionic sugar-containing polymers of bacterial cell walls including teichoic acids are considered a useful and promising class of chemical compounds. These polymers exhibit structural diversity, play an important role in vital cellular processes, and are widely distributed among Gram-positive bacteria [1]. Studies of the genera Nocardiopsis [2] and Glycomyces [3] and of a number of phenotypic clusters of the genus Streptomyces [4] have shown that the species of this group of actinomycetes differ considerably in the composition and structure of their anionic sugar-containing polymers [5].
The goal of this work was to analyze in more detail the structure of anionic polymers in order to reveal their taxonomic specificity for additional groups of actinomycetes.
We studied the cell walls of four streptoverticille strains, representatives of two genospecies of a historically isolated group of the Streptomyces genus characterized by the verticillate type of hyphal branching [5].
MATERIALS AND METHODS
Streptomyces hachijoensis VKM Ac-191T (= NRRL B-3106T) and S. cinnamoneus subsp. azacoluta VKM Ac-606T (= NRRL B-1699T) strains, which belong to one genospecies (DNA--DNA homology of 81%) and the members of another genospecies, S. biverticillatus VKM Ac-891T (= NRRL ISP-5272T) and S. baldaccii VKM Ac-821T (= NRRL B-3500T), with DNA homology of 77% [5] were used in this study.
The cultures were grown in flasks containing 100 ml of yeast peptone medium (YPM) [6] on shakers at 28°C. Thoroughly homogenized two-day cultures grown in the same medium were used as inoculum. The biomass was collected at the logarithmic phase (20-24 h), washed with distilled water, and frozen.
Cell walls were obtained by disruption of mycelium suspended in water containing 1% SDS with an ultrasound disintegrator, heating of the homogenate at 100°C for 5 min, and subsequent differential centrifugation [7]. The cell wall preparations were lyophilized.
Isolation of polymers. The cell walls were treated with 10% trichloroacetic acid for 48 h at 4°C, the extract was separated from cell debris by centrifugation, the pellet was washed with water, and pooled extracts were dialyzed against distilled water and lyophilized.
Acid hydrolysis. To identify phosphoesters, polyols, and amino sugars, the cell walls and polymers were hydrolyzed in 2 M HCl for 3 h at 100°C. To determine the component ratio in phosphoesters, hydrolysis was carried out in 6 M HCl for 4 h at 100°C.
Enzymatic hydrolysis with phosphomonoesterase (EC 3.1.31; Sigma, USA) was conducted in ammonium acetate buffer, pH 9.8, at 37°C for 18 h.
Electrophoresis and chromatography were carried out using Filtrak FN-13 paper. To separate phosphoesters, amino sugars, and diamino sugars, electrophoresis was performed in pyridine acetate buffer, pH 5.6, at 20 V/cm for 3-4 h. To separate glycerol and amino sugars, descending chromatography in pyridine--benzene--butane-1-ol--water (3:1:5:3 v/v) was employed.
Reagents used for the detection of specific classes of compounds were as follows: the Isherwood reagent for phosphoesters [8]; 5% aqueous AgNO3 for glycerol and amino sugars; amino sugars were stained with ninhydrin.
Phosphorus was assayed as described in [9]; glucosamine was assayed as described in [10].
Molecular mass was determined on a Sephadex G-50 column using teichoic acids with known molecular masses as markers [11].
NMR spectra were acquired from 2-3% solutions in D2O at 30°C with a Bruker DRX-500 instrument (Bruker, Germany). Acetone was used as standard (2.225 ppm in 1H- and 31.45 ppm in 13C-spectra, respectively). Two-dimensional spectra were obtained using a Bruker software package.
RESULTS AND DISCUSSION
The cell walls of Streptomyces hachijoensis VKM Ac-191T and Streptomyces cinnamoneus subsp. azacoluta VKM Ac-606T contained 2.5% phosphorus, this indicating the presence of an anionic polymer, and did not contain monosaccharides. The polymer was extracted from the cell walls with 10% trichloroacetic acid and characterized. Identical results were obtained with both actinomycetes (see below).
Mild hydrolysis (2 M HCl, 3 h, 100°C) of the polymer produced two phosphoesters. One of them (predominant, E1) migrated to the cathode during electrophoresis, this being unusual for phosphate-containing esters. The ester was isolated by preparative paper electrophoresis and analyzed. Phosphomonoesterase cleaved all phosphate groups of the E1 ester, this being consistent with phosphomonoester structure. After acid hydrolysis in 6 M HCl for 4 h at 100°C, only glucosamine was identified. In the E1 ester, the P:GlcN ratio was 1:2. These data indicate linkage between phosphate and disaccharide groups. The cationic behavior of the ester suggested that both amino groups of amino sugars were free.
Considering the NMR spectroscopic data (see below), we suggest that E1 is a 6'-phosphate of 2-amino-2-deoxy-alpha-D-glucopyranosyl-(1-->6)-2-amino-2-deoxy-D-glucopyranose.
The second (minor) phosphoester (E2) was neutral during electrophoresis; it is a phosphomonoester containing phosphorus and glucosamine in 1:1 ratio.
Accordingly, we suppose that E2 is the 6-phosphate of 2-amino-2-deoxy-D-glucopyranose (see NMR spectroscopic data).
The polymer was further analyzed by NMR spectroscopy.
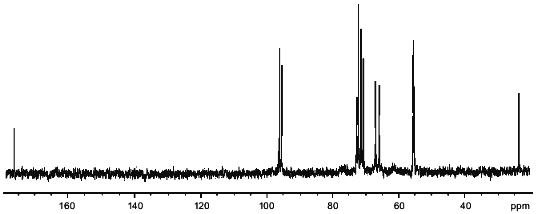
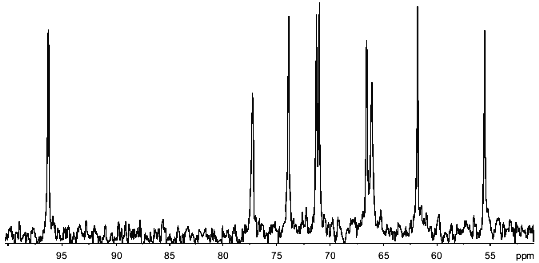
13C-NMR of the polymers produced 14 signals (Fig. 1). Twelve of these were in the region characteristic of amino sugars. Two signals of equal intensity were in the region of anomeric carbon atoms (96.15 and 95.55 ppm), and two other signals with chemical shifts of 55.3 and 55.55 ppm were typical of carbon bonded to nitrogen. An attached-proton test (APT [12]) revealed signals at 65.95 and 67.1 ppm from CH2O groups. The position of these peaks in the spectrum corresponded to substituted C-6 hexapyranose residues. For two carbons linked to nitrogen, only one set of signals from CH3CON groups (23.6 and 176.1 ppm, respectively) was observed. Some signals in the 13C-NMR spectrum were split into doublets (95.55, 55.3, 72.7 ppm), probably due to spin--spin interactions through two or three bonds with phosphorus.
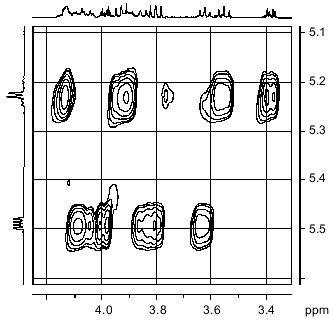
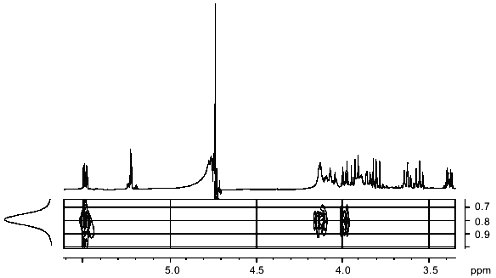
1H-NMR spectra has two main signals in the anomeric region (Table 1); one (5.490 ppm) is split into a doublet of doublets, and the other (5.227 ppm) is a doublet with a coupling constant of 3.5 Hz. In the resonance region of CH3CO groups, a singlet (2.066 ppm), whose intensity corresponds to three protons is observed. The spectrum was analyzed by COSY and TOCSY homonuclear two-dimensional spectroscopy (Fig. 2, Table 1). Analysis of the spectra showed that according to coupling constants, both sugars are in the alpha-gluco configuration. The high-field position of the H-2 signal of GlcpNH3+ (fragment A) (3.381 ppm) and the low-field position of the H-2 signal of GlcpNAc (fragment B) (3.988 ppm) indicates the presence of N-acetyl in fragment B and its absence in fragment A. As in the 13C-NMR spectrum, some proton signals are additionally split due to interactions through three and four bonds with phosphorus. The location of these signals in proton spectrum is clearly seen from the two-dimensional 1H/31P-spectrum (Fig. 3). Comparison of this spectrum with the COSY and TOCSY spectra shows that the additional splitting at phosphorus has H-1 and H-2 protons of fragment B and H-6.6' of fragment A. Consequently, fragments A and B are linked by a phosphodiester bond.Fig. 1. 13C-NMR spectrum of sugar-1-phosphate polymer of Streptomyces hachijoensis VKM AC-191 and Streptomyces cinnamoneus subsp. azacoluta VKM Ac-606.
Table 1. NMR-spectroscopic data of the
anionic polymer of cell walls of Streptomyces hachijoensis VKM
Ac-191T and Streptomyces cinnamoneus subsp.
azacoluta VKM Ac-606T (coupling constants with
phosphorus in Hz are given in parentheses)
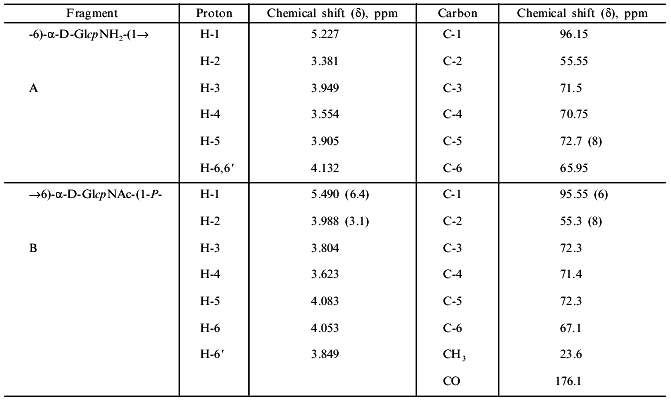
Fig. 2. Part of the TOCSY spectrum of the sugar-1-polymer of Streptomyces hachijoensis VKM AC-191 and Streptomyces cinnamoneus subsp. azacoluta VKM Ac-606.
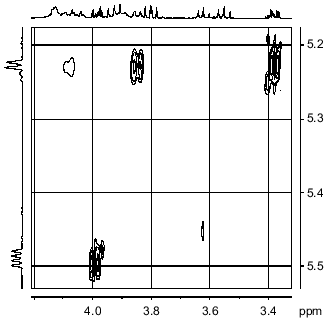
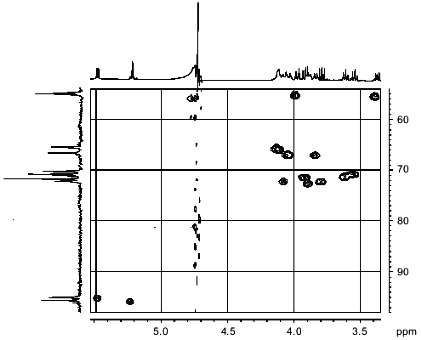
The NOESY spectrum of the polymer (Fig. 4) demonstrates that the anomeric proton of fragment B is spatially close only to its own H-2 proton, while the anomeric proton of fragment A, in addition to a cross peak with its own H-2 proton, shows cross peaks with H-6 and H-6' protons of fragment B.Fig. 3. Part of the 1H/31P-HMQC spectrum of the sugar-1-polymer of Streptomyces hachijoensis VKM Ac-191 and Streptomyces cinnamoneus subsp. azacoluta VKM Ac-606.
The 1H/13C-HMQC spectrum (Fig. 5) allowed us to assign all signals in the carbon spectrum and confirmed the types of substitutions in fragments A and B.Fig. 4. Part of the NOESY spectrum of the sugar-1-phosphate polymer of Streptomyces hachijoensis VKM Ac-191 and Streptomyces cinnamoneus subsp. azacoluta VKM Ac-606.
Thus, fragment A is linked by a 1-->6 glycoside bond with fragment B, forming a disaccharide repeating unit of a linear polymer. The phosphodiester bond links repeating disaccharide units involving C-1 of fragment B and C-6 of fragment A.Fig. 5. Part of the 1H/13C-HMQC spectrum of the sugar-1-phosphate polymer of Streptomyces hachijoensis VKM Ac-191 and Streptomyces cinnamoneus subsp. azacoluta VKM Ac-606.
The molecular mass of polymers averaged 9 kD, this corresponding to 20 repeating units.
The formation of E1 can be explained, first, by some instability of glycosyl phosphate bonds under acidic conditions [13] and, second, by the cleavage of the acetyl group of fragment B during mild acid hydrolysis (2 M HCl, 3 h, 100°C), while the 1-->6 glycoside bond between fragments A and B is relatively stable [14]. Partial hydrolysis leads to formation of the E2 ester.
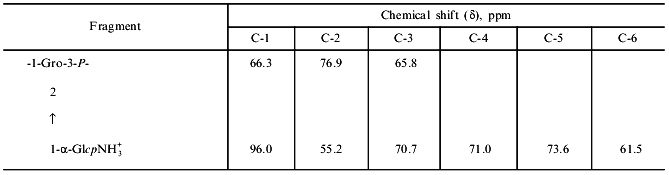
The cell walls of Streptomyces biverticillatus VKM Ac-891T and Streptomyces baldaccii VKM Ac-821T contained 2.6% phosphorus and had no neutral monosaccharides. During acid hydrolysis, some glycerol mono- and diphosphates were formed, this suggesting the presence of glycerol teichoic acids in the cell walls of these microorganisms. The polymer was isolated from the cell walls and analyzed. Since the teichoic acids found in both streptoverticille species were identical, the results described below refer to both polymers.
Analysis of the polymer by electrophoresis at pH 5.6 (with subsequent staining with the Isherwood reagent and ninhydrin) revealed its neutral properties, this being quite unusual. The polymer was not hydrolyzed by alkali and produced a limited amount of glycerol mono- and diphosphates and glucosamine upon acid hydrolysis. These data indicate that the teichoic acid is 1,3-poly(glycerol phosphate) in which most glycerol phosphate units are substituted at OH-2 by nonacetylated glucosamine. This could account for the stability of this teichoic acid to acid hydrolysis. It is known that glycosides of 2-amino-2-deoxysugars are extremely stable to acid hydrolysis [14]. The absence of a free OH-2 group in glycerol interferes with cleavage of the 1,3-poly(glycerol phosphate) chain [15].
The 13C-NMR spectrum of the teichoic acid revealed nine peaks of equal integral intensity (Fig. 6, Table 2). The spectrum was assigned to 1,3-poly(glycerol phosphate) polymer completely substituted at C-2 of glycerol by alpha-glucosamine.
The molecular mass of the polymers is 10-11 kD, this corresponding to 22-24 repeating units.
Table 2. 13C-NMR spectroscopic
data of teichoic acid from cell walls of Streptomyces
biverticillatus VKM AC-891T and Streptomyces
baldaccii VKM Ac-821T
Thus, our study of two streptoverticille genospecies (two strains in each) showed variation in phosphorus-containing polymers of cell walls. In Streptomyces hachijoensis VKM Ac-191 and Streptomyces cinnamoneus subsp. azacoluta VKM Ac-606, which belong to one genospecies on the basis of DNA--DNA hybridization, this is an anionic polymer containing disaccharide repeating units of 2-amino-2-deoxy-alpha-D-glucopyranosyl-(1-->6)-2-acetamido-2-deoxy-alpha-D-glucopyranose linked at C-1 and C-6' by phosphodiester bonds. The cell walls of Streptomyces biverticillatus VKM Ac-891 and Streptomyces baldaccii VKM Ac-821, assigned to a different genospecies, contain a teichoic acid, 1,3-poly(glycerol phosphate), completely substituted by glucosamine.Fig. 6. 13C-NMR spectrum of teichoic acid from the cell walls of Streptomyces biverticillatus VKM Ac-891T and Streptomyces baldaccii VKM Ac-821T.
It is of particular interest that, in both instances, despite the presence of phosphate groups in each repeating unit, the polymers were neutral. The explanation could be as follows. Since each repeating unit contains a glucosamine residue with a free amino group, it neutralizes the negative charge of an adjacent phosphate group [16]. The polymers described above have not been previously found in bacteria.
This work was supported in part by INTAS (grant No. 96-1571), the Russian Foundation for Basic Research (grant No. 98-04-49277), and the "Universities of Russia" Program.
REFERENCES
1. Naumova, I. B., and Shashkov, A. S. (1997)
Biochemistry (Moscow), 62, 809-840.
2. Streshinskaya, G. M., Tul'skaya, E. M., Shashkov,
A. S., Evtushenko, L. I., Taran, V. V., and Naumova, I. B. (1998)
Biochemistry (Moscow), 63, 191-194.
3. Potekhina, N. V., Tul'skaya, E. M., Shashkov, A.
S., Taran, V. V., Evtushenko, L. I., and Naumova, I. B. (1998)
Mikrobiologiya, 67, 404-408.
4. Tul'skaya, E. M., Shashkov, A. S., Evtushenko, L.
I., Bueva, O. V., and Naumova, I. B. (1997) Biochemistry,
62, 289-293.
5. Labeda, D. P. (1996) Int. J. Syst.
Bacteriol., 46, 699-703.
6. Naumova, I. B., Kuznetsov, V. D., Kudrina, K. S.,
and Bezzubenkova, A. P. (1980) Arch. Microbiol., 126,
71-75.
7. Streshinskaya, G. M., Naumova, I. B., and Panina,
L. I. (1979) Mikrobiologiya, 48, 814-819.
8. Benson, A. (1960) Biochemical Methods in Plant
Analysis [Russian translation], Izd-vo Inostrannoi Literatury,
Moscow, pp. 146-182.
9. Weil-Malherbe, H., and Green, R. H. (1951)
Biochem. J., 49, 286-292.
10. Gladyshev, B. N. (1956) Biokhimiya,
21, 227-230.
11. Tul'skaya, E. M., Streshinskaya, G. M., Naumova,
I. B., Shashkov, A. S., and Terekhova, L. A. (1993) Arch.
Microbiol., 160, 299-305.
12. Patt, S. L., and Shoolery, I. N. (1995) J.
Magn. Reson., 46, 535-539.
13. Leloir, L. F., and Cardini, C. E. (1957)
Meth. Enzymol., 3, 840.
14. Foster, A. B., Horton, D., and Stacey, M. (1957)
J. Chem. Soc., 81-92.
15. Kelemen, M. V., and Baddiley, J. (1961)
Biochem. J., 80, 246-254.
16. Baddiley, J., Hancock, I. C., and Sherwood, P.
M. A. (1973) Nature, 243, 43-45.