Preparation and Characterization of Microencapsulated Proteinase Inhibitor Aprotinin
N. V. Larionova1, N. F. Kazanskaya1, N. I. Larionova1*, G. Ponchel2, and D. Duchene2
1School of Chemistry, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-5417; E-mail: nilar@enzyme.chem.msu.ru2Laboratory of Physico-Chemistry, Pharmacotechnique, and Biopharmacy, UMR CNRS 8612, School of Pharmacy, University of Paris-Sud, 92290 Chatenay-Malabry, France
* To whom correspondence should be addressed.
Received April 8, 1999
Preparation of microcapsules through interfacial cross-linking of soluble starch/hydroxyethyl starch and bovine serum albumin (BSA) with terephthaloyl chloride is described. The proteinase inhibitor aprotinin, either native or active site protected, was microencapsulated, being incorporated in the aqueous phase. The influence of aqueous phase pH, BSA, and terephthaloyl chloride concentrations as well as stirring rate on microcapsule morphology and size was studied. The polycondensation pH was shown to be the determining factor for tough microcapsule production with a high encapsulation yield. The size of the microcapsules ranged between 10-30 and 50-100 µm at stirring speed 1500 and 500 rpm, respectively. Fourier transform infrared spectroscopic studies were performed on microcapsules prepared under various conditions. A correlation was established between spectral changes and microcapsule morphology and size. The optimal conditions for microcapsule degradation by alpha-amylase were found. Active site-protected aprotinin was shown to fully retain its activity after microencapsulation.
KEY WORDS: microencapsulation, proteinase inhibitor, aprotinin, starch, biodegradation
Protein proteinase inhibitors have been long used for the treatment and prophylaxis of a variety of severe disorders caused by uncontrollable activation of serine proteinases [1, 2]. One of the essential drawbacks of the protein proteinase inhibitors, like other protein and peptide drugs, is the necessity of parenteral administration [3]. Recently, extensive research has been done to find alternative routes of protein drug delivery (oral, intranasal, etc.) [4, 5]. Oral administration of proteins is the most convenient. However, the bioavailability of proteins delivered by this route is rather poor due to their hydrolysis and enzymatic degradation in the gastrointestinal tract [6].
Microparticles are considered to be a promising system for oral protein drug delivery because of they ensure physical protection to the encapsulated proteins against inactivation during the gastrointestinal transit [7]. Besides, microparticles can manipulate drug release kinetics, achieving optimal therapeutic effect [7].
Starch microspheres are a carrier suitable for the development of protein delivery systems due to their biocompatability, shelf-life stability, high loading capacity, biodegradability, and controlled release of the encapsulated drug.
The objective of this study was to develop a method for entrapment of the proteinase inhibitor aprotinin in microcapsules prepared from cross-linked soluble starch/hydroxyethyl starch and BSA, and to investigate the influence of manufacturing conditions on the morphology and structure of the microcapsules as well as the aprotinin activity.
MATERIALS AND METHODS
Soluble starch (Glucidex 2) was supplied by Roquette Freres (France). Hydroxyethyl starch (Volekam) was obtained from NIOPIK (Russia), weight-average molecular weight 200 kD, substitution degree 0.6. Terephthaloyl chloride was from Aldrich-Chimie (France). Surfactants used were sorbitan trioleate (Span 80) and polyoxyethylenesorbitan trioleate (Tween 85) from ICI (Germany). Chloroform, cyclohexane, and ethanol were from Prolabo (France). BSA, bovine trypsin with 50% active protein content [8], esterase (19 IU/mg) from porcine liver, alpha-amylase (27 U/mg), and pancreatin from porcine pancreas were all from Sigma (USA). Aprotinin (Gordox) was obtained from Gedeon Richter (Hungary). N-Benzoyl-L-arginine-p-nitroanilide was from Sigma.
Preparation of starch microcapsules. Microcapsules were prepared from soluble starch or hydroxyethyl starch, and protein using our modification [9] of the interfacial cross-linking technique proposed by Levy et al. [10] for polysaccharides. Varying amounts of soluble starch/hydroxyethyl starch and bovine serum albumin were dissolved in the selected buffer. Then the aqueous phase was emulsified in cyclohexane (1:3 v/v) containing 5% (v/v) Span 80. After 15 min a terephthaloyl chloride solution in chloroform was added to the emulsion (1:2.4 v/v). Stirring was continued for 30 min. The microparticles were washed with cyclohexane (twice), with 2% (v/v) Tween 85 solution in ethanol, with 95% ethanol (three times), and with distilled water (twice). Finally, the microcapsules were resuspended in water and lyophilized.
Variations were introduced in the stirring rate and the composition of the aqueous phase on the microcapsule preparation. Phosphate buffers (0.1 M) were used at pH 5.0, 6.0, and 7.0, and 0.5 M carbonate buffers were used at pH 8.0 and 9.8. Concentrations of starch and BSA were 10 and 0-5%, respectively. Terephthaloyl chloride concentrations were 2, 3, and 5%. All batches were prepared at least three times.
Microencapsulation of the proteinase inhibitor. Aprotinin was used for microcapsulation either in the native form or in a modified form, where amino groups were protected by citraconic anhydride [11]. The inhibitor in various concentrations (0.4 and 0.8%) was incorporated in the aqueous phase during the encapsulation.
Further treatment of microcapsules. Microcapsule samples prepared as described above were divided into two parts before lyophilization. One part of the microcapsules was soaked for 8 h in 0.1 M carbonate buffer, pH 8.0, at room temperature, and the second part was kept untreated and used as a control. If the microcapsules contained citraconylated inhibitor, the microspheres were additionally incubated for 5 h at pH 2.0 for the removal of the protection. Then they were rinsed three times with distilled water and lyophilized.
Microcapsule characterization. Microcapsule morphology was studied by optical microscopy and scanning electron microscopy. Microcapsules were sized using a Coulter Multisizer II, Sampling Stand II A (United Kingdom). Size distribution was displayed in terms of volume against particle size.
Infrared spectra. The samples for study on IR-spectra were prepared according to the standard technique: 10 mg of lyophilized microcapsules was ground with 200 mg of KBr. The mixture was compressed in tablets, 1 mm thick, under a pressure of 10 kPa. The Fourier transform IR-spectra were obtained with an Impact 420 spectrometer (United Kingdom).
Enzymatic degradation of microcapsules in vitro. Lyophilized microcapsules (10 mg) were resuspended in 5 ml of 0.1 M carbonate buffer (pH 8.0) containing either a suitable amount of alpha-amylase or a mixture of esterase, alpha-amylase, and pancreatin. The sample was incubated at 37°C with agitation at 40 rpm. The antitrypsin activity was measured in aliquots withdrawn from the supernatant at appropriate time intervals.
Determination of protein content. The protein content in the aliquots was quantified by the method of Lowry et al. [12]. The protein concentration in the enzymatic solution used for the microcapsule degradation was taken into account on calculation of amount of protein released from the microcapsules.
Determination of antitrypsin activity. The antitrypsin activity in solution obtained after the enzymatic degradation of aprotinin-containing microcapsules was assayed by measuring the inhibition of the amidase activity of bovine trypsin [13]. For this, 0.2 ml of the solution under investigation was mixed with 0.2 ml of trypsin solution (50 µg/ml). The reaction mixture volume was adjusted to 2.4 ml with 0.05 M Tris buffer (pH 8.0), and the mixture was incubated for 5 min. Then 0.1 ml of N-benzoyl-L-arginine-p-nitroanilide solution (10.8 mg/ml) in DMSO was added in the sample. The mixture was incubated for 15 min, then the reaction was stopped by adding 0.5 ml of 5 M acetic acid. The residual trypsin activity was determined by reading absorbance at 405 nm against the control, which did not contain aprotinin.
RESULTS AND DISCUSSION
Morphology and size of microparticles. A series of experiments showed the dependence of morphology of the microparticles prepared from soluble starch or hydroxyethyl starch on aqueous phase pH and cross-linking agent concentration. Microparticles from hydroxyethyl starch were not formed, but ones from soluble starch were obtained with low yield when phosphate buffers (pH 5.0, 6.0, and 7.0), as well as carbonate buffer (pH 8.0) were used. By optical microscopy, the particles appeared fragile and nonuniform and formed aggregates. Increasing the cross-linking agent concentration as well as the reaction time did not effect the particle morphology.
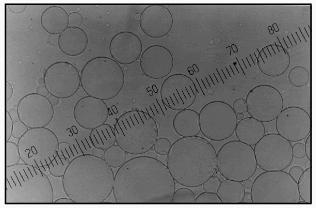
The use of 0.5 M carbonate buffer (pH 9.8) for aprotinin encapsulation in microparticles from soluble starch resulted in formation of transparent, uniform, non-aggregated spherical particles (Fig. 1). The hydroxyethyl starch particles prepared using the same conditions looked less firm and nonhomogeneous in size. Strengthening microcapsule walls in both cases and an increase in microcapsule storage duration was achieved by increasing terephthaloyl chloride concentration to 5%.
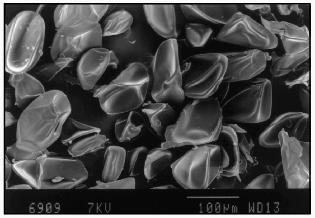
The particles had a smooth surface as scanning electron microscopy demonstrated (Fig. 2). The form of lyophilized particles (collapsed sphere) showed the preparation of microcapsules or particles of reservoir-type. The microcapsules prepared as described above were not damaged by lyophilization and easily recovered their spherical shape after rehydration in a buffer medium.Fig. 1. Optical microphotograph of microcapsules prepared from 10% solution of soluble starch, 5% solution of BSA, and 0.4% solution of aprotinin in buffer, pH 9.8, using 2% terephthaloyl chloride. Magnification 20.
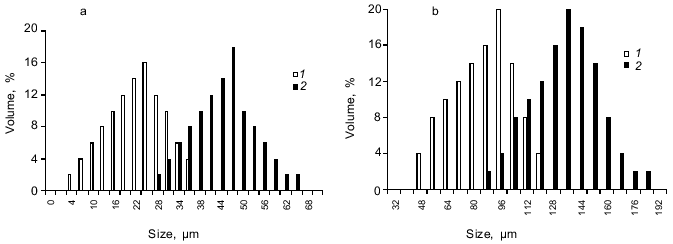
The microcapsule size could be adjusted by varying the stirring speed. For example, the microcapsule size prepared from 10% starch, 1 or 5% BSA, and 0.4% aprotinin at 2-% terephthaloyl chloride concentration could range from 10-30 µm for agitation at 1500 rpm to 50-100 µm for the stirring speed 500 rpm (Fig. 3, a and b).Fig. 2. Scanning electron micrograph of lyophilized aprotinin loaded microcapsules prepared as described in the legend to Fig. 1.
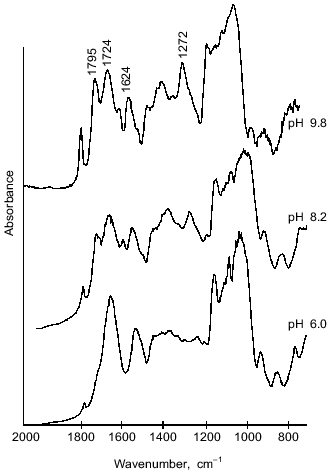
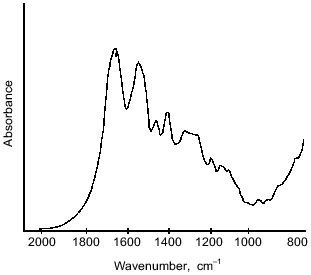
The structure of microcapsule membrane according to IR-spectroscopy. The processes proceeding on preparation of microcapsules were studied by IR-spectroscopy. The IR-spectra of microcapsules prepared from 10% soluble starch or hydroxyethyl starch, 5% BSA, and 0.4% aprotinin through interfacial cross-linking at various pH values are presented in Fig. 4. The absorption bands at 3650-3200 cm-1 caused by O--H valent vibrations in polysaccharides were not considered, since the most important notable spectral changes were found in the 2000-800 cm-1 range. The intense bands characterizing the protein component (amide I band in the 1690-1600 cm-1 region, amide II band at 1545 cm-1, and amide III band in the 1300-1230 cm-1 region [14]) were notable in the spectra of microcapsules.Fig. 3. Dependence of size distribution of the starch microcapsules (10% starch, 1% BSA, 0.4% aprotinin, 2% terephthaloyl chloride, pH 9.8) on the stirring speed: a) stirring speed 1500 rpm (1), 1000 rpm (2); b) stirring speed 500 rpm: 1) without treatment; 2) capsules treated with buffer (pH 8.0) for 8 h.
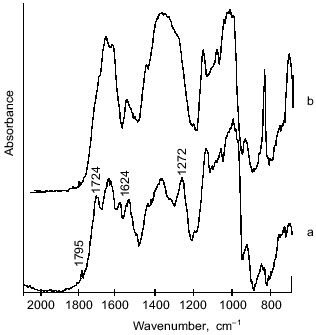
However, there are considerable differences between the microcapsule spectra (Fig. 4) and the spectrum of BSA (Fig. 5). First, a band at 1795 cm-1 appeared, which was assigned to the asymmetrical C=O stretching vibrations of anhydrides [14, 15]. A second important change in the spectrum was observed at 1724 cm-1, a band attributed to the C=O stretching vibrations of aromatic acid esters [14, 15]. It should be noted that the intensities of these peaks increased on increasing the pH value of cross-linking with terephthaloyl chloride. On the other hand, one of the components of the amide I band with maximal absorbance at 1624 cm-1, corresponding to beta-sheet content, was significantly increased with intensification of protein modification on increasing the pH value.Fig. 4. IR-spectra of microcapsules prepared at various pH values from 10% solution of starch derivatives, 1% BSA, and 0.4% aprotinin with 5% terephthaloyl chloride. Soluble starch (pH 6.0 and 8.2), hydroxyethyl starch (pH 9.8).
Further, changes in the shape and area of the amide III band occurred in the IR-spectra of microcapsules. Although detailed interpretation of this region is difficult because overlapping the amide III band and bands caused by in-plane deformational modes of O--H bond (1450-1250 cm-1) [14], as well as C--O valent vibrations of esters (1330-1050 cm-1) [14] and of aromatic anhydrides (1282-1220 cm-1) [15]; nevertheless some assignments of bands can be made. So, a marked increase in the peak area with maximum at 1272 cm-1 can be attributed to formation of terephthalic esters, by analogy with the paper of Levy et al. [15] devoted to investigation of human serum albumin microcapsules. Moreover, in the 1200-1000 cm-1 range, the microcapsule spectra (Fig. 4) reveal intense bands which may be derived from C--O valent vibrations in polysaccharides [14]. Some overlapping is possible with absorption bands at 1172 and 1040 cm-1, which can be assigned to ester groups as shown by Levy et al. [15].Fig. 5. IR-spectrum of BSA in the 2000-800 cm-1 range.
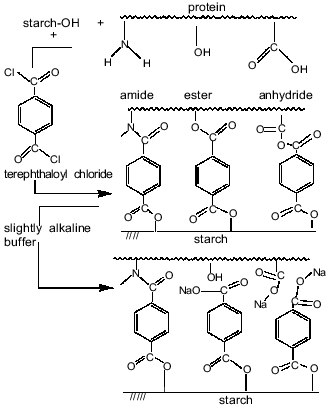
Thus, the results indicated the involvement of starch hydroxy groups and various functional groups of protein in the polycondensation reaction on the increase in aqueous phase pH. Figure 6 schematically represents cross-linking of polysaccharide and protein macromolecules with terephthaloyl chloride. At low pH values, the microcapsule wall is formed via acylation, on one hand, of starch OH-groups and, on the other hand, mainly of amino groups and in the lesser extent of carboxylate groups of the protein. However, the formation of new amide bonds did not affect the amide bands of the microcapsule spectrum. On increasing pH value, the progressive acylation of carboxylic groups and hydroxy groups of serine, threonine, and tyrosine residues of protein was observed. As the result of forming a variety of intermolecular links, the protein structure in the membrane became more ordered; an increase in beta-sheet content (1624 cm-1) showed this.
The proposed microcapsule membrane structure was confirmed by the data on changes in morphology, size, and IR-spectrum of the microcapsules prepared at pH 9.8 after their additional treatment with a slightly alkaline buffer. Soaking microcapsules in pH 8.0 buffer for 8 h resulted in twofold increase in their size (Fig. 3b). The peak at 1795 cm-1, corresponding to anhydrides, was not observed in the spectrum of the treated microcapsules (Fig. 7). The 1724 cm-1 peak attributed to esters converted to a shoulder, while the 1272 cm-1 peak, characteristic for terephthalic acid phenyl esters, disappeared. The data show that the hydrolysis of anhydride bonds, which are the most labile in slightly alkaline medium, and some of the ester bonds, involving tyrosine residues, results in the morphological changes of the microcapsules and increase of their size.Fig. 6. Scheme of starch and protein binding with terephthaloyl chloride and of treatment of capsules with a buffer (pH 8.0).
The membrane of microcapsules treated with a slightly alkaline buffer becomes more flexible due to a lower number of cross-links. The protein in the membrane structure becomes less ordered, as indicated in the disappearance of beta-sheet (1624 cm-1 band). The indicated changes in the structure of the microcapsule wall affect the velocity of enzymatic degradation of the microcapsules.Fig. 7. Effect of treatment of microcapsules (prepared from 10% soluble starch, 1% BSA, 0.8% aprotinin with 2% terephthaloyl chloride) on their IR-spectrum: a) no treatment; b) microcapsules treated with buffer (pH 8.0) for 8 h.
Study of the release of active aprotinin on enzymatic degradation of microcapsules. The susceptibility of microcapsules to degradation by a number of enzymes catalyzing the disruption of bonds involved in microcapsule wall formation was studied. The microcapsules were found to be resistant to esterase solution (19 IU/ml).
On the contrary, alpha-amylase (0.2-1 mg/ml) was the most destructive for the microcapsules; the use of pancreatin (1 mg/ml) and esterase (1 mg/ml) in addition to alpha-amylase only very slightly enhanced the rate of microcapsule dissolution. Poor microcapsule degradability by a mixture of proteolytic enzymes suggested low BSA content in the microcapsule wall, which was connected with the relatively low BSA concentration (1-5%) in the aqueous phase during the microparticle preparation.
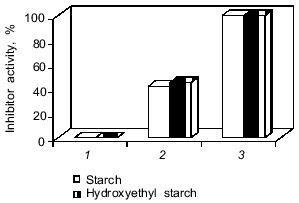
The protein was released from the capsules only after the enzymatic degradation of their walls. Leakage of protein from the capsules was not observed on incubation in buffer. However, we failed to detect antitrypsin activity in the medium (Fig. 8, bar 1) despite the 45% protein release from the microcapsules by alpha-amylase for 6 h. The absence of the inhibitor activity and the comparative resistance of the microcapsule wall to the enzymatic degradation could be the consequence of the extensive cross-linking of soluble polysaccharides and proteins which created steric hindrance of the effective interactions of enzymes with macromolecular substrates (protein and starch) and with the protein inhibitor. To decrease the cross-linking degree of starch derivatives and proteins, the microcapsules were treated with a slightly alkaline buffer. This resulted in total release of protein and detection of 45% encapsulated inhibitor activity of native aprotinin after alpha-amylase action (Fig. 8, bar 2). Modification of the inhibitor active site amino group (Lys-15) may account for inactivation of half of the aprotinin molecules. To prevent acylation of the aprotinin amino groups by terephthaloyl chloride, aprotinin with citraconylated (reversibly protected) amino groups was used for encapsulation. This revealed 100% antiproteinase activity of aprotinin after degradation of capsules by alpha-amylase.
Thus, the procedure proposed for microcapsulated aprotinin manufacturing ensures production of a preparation that is stable on storage. The preparation totally retained the biological activity of the proteinase inhibitor and was able to release the inhibitor in a time period comparable with gastrointestinal transit.Fig. 8. Release of inhibitor activity after degradation of microcapsules by alpha-amylase (0.2 mg/ml), 37°C, for 6 h. The microcapsule composition is indicated in the legend to Fig. 3b. 1, 2) Native aprotinin; 3) citraconylated aprotinin. The microcapsules were treated with buffer (pH 8.0) before enzymatic degradation (2, 3).
The authors thank Dr. N. A. Moroz for the preparation of the manuscript for publication.
This work was supported by a grant from the Ministry of Science and Technologies of the Russian Federation and by NATO Linkage grant No. 960962.
REFERENCES
1.Schnebli, H. P., and Braun, N. J. (1986) in
Proteinase Inhibitors (Barrett, A. J., and Salvesen, G., eds.)
Elsevier Science, Amsterdam, pp. 613-627.
2.Veremeenko, K. N., Goloborod’ko, O. P., and
Kizim, A. I. (1988) Proteolysis in Norm and Pathology [in
Russian], Zdorov’e, Kiev.
3.Fritz, H., and Wunderer, G. (1983)
Arzneim-Forsch., 33, 479-494.
4.Lee, V. H. L., Dodda-Kashi, S., Grass, O. M., and
Rubas, W. (1991) Peptide and Protein Drug Delivery (Lee, V. H.
L., ed.) Marcel Dekker, New York, pp. 691-738.
5.Talmadge, J. E. (1993) Adv. Drug Deliv.
Rev., 10, 247-299.
6.Story, M. J. (1991) in Peptides. Theoretical and
Practical Approach to Their Delivery, Capsugel Library Symposia
Series, USA, pp. 55-67.
7.Kissel, T., and Koneberg, R. (eds.) (1996)
Microparticulate Systems for the Delivery of Proteins and
Vaccines.
8.Chase, T., and Shaw, E. (1967) Biochem. Biophys.
Res. Commun., 29, 508-514.
9.Larionova, N. V., Moroz, N. A., Larionova, N. I.,
Hamdi, G., Dumistracel, I., Duchene, D., and Ponchel, G. (1997)
Proc. Symp. "Particulate Systems, From Formulation to
Production", pp. 91-92.
10.Levy, M.-C., and Andry, M.-C. (1990) Int. J.
Pharm., 62, 27-35.
11.Larionova, N. I., Kazanskaya, N. F., and
Sakharov, I. Yu. (1977) Biokhimiya, 42, 1237-1243.
12.Lowry, O. H., Rosenbrough, N. J., Farrand, A. L.,
and Randall, R. J. (1951) J. Biol. Chem., 193,
265-275.
13.Kakade, M. L., Simons, N., and Liener, I. E.
(1969) Cereal Chem., 46, 518-526.
14.Brown, D. W., Floyd, A. J., and Sainsbury, M.
(1992) Organic Spectroscopy [Russian translation], Mir,
Moscow.
15.Levy, M.-C., Lefebvre, S., Rahmouni, M., Andry,
M.-C., and Manfait, M. (1991) J. Pharm. Sci., 80,
578-585.