Different Pathways for Mitogenic and Enzyme Induction Signal Transduction by Cytochrome P450 Inducers
E. L. Gujaeva, N. M. Ostashkina, V. F. Kondalenko, and V. A. Koblyakov*
Blokhin Cancer Research Center, Russian Academy of Medical Sciences, Kashirskoe Shosse 24, Moscow, 115478 Russia; fax: (095) 324-1205* To whom correspondence should be addressed.
Received December 10, 1998; Revision received March 25, 1999
The correlation between enzyme induction and cell proliferation caused by inducers of xenobiotic metabolizing enzymes was studied using a cell culture expressing a constitutive level of cytochrome P450 (hepatoma McA RH 7777) and a cell culture in which cytochrome P450 was absent (hepatoma 27). In hepatoma 27 cells, the inducers did not induce the synthesis of xenobiotic metabolizing enzymes but stimulated cell proliferation. Thus, the processes of signal transduction for enzyme induction and for cell proliferation by the inducers are different.
KEY WORDS: induction, cytochrome P450, proliferation, polycyclic aromatic hydrocarbons, phorbol esters
Abbreviations: BA) benz[a]anthracene; BP) benzo[a]pyrene; BPH) BP-hydroxylase; alpha-NF) alpha-naphthoflavone; beta-NF) beta-naphthoflavone; 7-EROD) 7-ethoxyresorufin deethylase; PAH) polycyclic aromatic hydrocarbons; PCMB) p-chloromercuribenzoate; PKC) protein kinase C; TPA) 12-O-tetradecanoylphorbol 13-acetate; PT) phenanthrene; DMF) dimethylfumarate.
The enzymatic metabolism of lipophilic xenobiotics into water-soluble
compounds can be divided into two phases. Phase I includes oxidation
catalyzed by a monooxygenase enzyme system where cytochrome P450 plays
the key role. Phase II includes the conjugation or detoxication of the
active oxygen species-containing metabolite. Phase II is catalyzed by
glutathione-S-transferase, UDP-glucuronyltransferase, NADP(H)-quinone
oxidoreductase, epoxidase, etc. Enzymes of phases I and II of
xenobiotic metabolism can be induced. Inducers are divided into mono-
and bifunctional types: monofunctional inducers induce only the enzymes
of phase II, and bifunctional inducers induce both phases of metabolism
[1, 2]. The mechanisms of
action of mono- and bifunctional inducers are different. Certain PAH,
beta-NF, halogenated dioxins, etc. are bifunctional inducers.
Bifunctional inducers are agonists of Ah-receptors, and the
ligand--receptor complex interacts with DNA at xenobiotic-responsive
elements (XRE) in the regulatory regions of the genome encoding certain
isoforms of cytochrome P450 and enzymes of phase II of xenobiotic
metabolism. The list of monofunctional inducers includes a number of
hydrophilic compounds with electrophilic or phenolic moieties.
Induction by these compounds is mediated via the so-called
electrophilic or antioxidant responsive elements (ARE) located in the
regulatory regions of the genes [3, 4]. The mechanism of signal transduction from inducers
to ARE is unknown. The process does not involve the Ah-receptor because
in hepatoma cells with defective Ah-receptor, NADP(H)-quinone
oxidoreductase is induced by monofunctional inducers [1]. Apart from stimulation of xenobiotic-metabolizing
enzyme synthesis, bifunctional inducers are mitogens in liver cells [5, 6]. Stimulation of
proliferation can be associated with cytochrome P450 function [7], but certain data suggest that bifunctional
inducers activate the immediate response genes (fos, jun)
and Ca2+ transport independently of cytochrome P450 [8].
The goal of the present work was to compare the mitogenic and inducing activity of mono- and bifunctional inducers in hepatoma cells which express (McA RH 7777) and do not express (hepatoma 27) cytochrome P450. Previously, we demonstrated [9] that hepatoma 27 cells transplanted subcutaneously do not express cytochrome P450 isoforms and cannot induce the enzyme, but following intrahepatic transplantation, a low constitutive level of the enzyme is detected and bifunctional inducers induce the corresponding cytochrome P450 isoforms. We assumed that expression of cytochrome P450 isoforms in hepatoma 27 cells grown on a glass or plastic surface would be similar to the cells transplanted subcutaneously.
A culture where xenobiotic-metabolizing enzymes cannot be induced can help to answer a question concerning the similarity or difference in transduction of mitogenic and inducing signals by inducers. The hepatoma McA RH 7777 cells were used as the system capable of induction of cytochrome P450 isoforms and the enzyme of phase II of metabolism.
MATERIALS AND METHODS
The hepatoma 27 and McA RH 7777 cells were grown in DMEM + RPMI medium containing 5% fetal calf serum. BPH was assayed by formation of 3-hydroxy-BP [10] after cell lysis by freezing--thawing; 7-EROD was assayed by formation of resorufin directly in the cell culture [11]. PAH were added to the culture at the dose of 5 µg/ml (acetone solution). beta-NF and alpha-NF were also dissolved in acetone and were added to the culture to make final concentrations of 1 and 50 µM, respectively. Final concentrations of PCMB and dimethylfumarate dissolved in the medium were 40 and 50 µM, respectively. Toxicity of BP was determined by the MTT test [12]. NADP(H)-quinone oxidoreductase was assayed by reduction of 2,6-dichlorophenolindophenol [13] at 25°C. Mitogenic effects were evaluated by counting the number of mitoses. Cells were cultured until they formed a monolayer and then incubated in the absence of serum for 5-7 days to completely suppress proliferative activity. Then, the tested compound was added in BSA. After 2 days, the cells were fixed with 4% formaldehyde and stained with hematoxylin--eosin. The number of mitoses was counted in each dish in five different regions. The means of five experiments are presented.
RESULTS
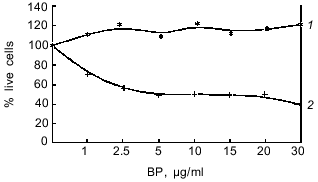
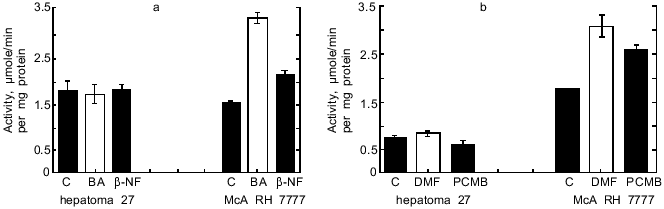
As seen from Table 1, in hepatoma McA RH 7777 cells a constitutive level of monooxygenase activity was revealed using BPH and 7-EROD, and this activity was enhanced by the bifunctional inducer BA. In the hepatoma 27 cells, constitutive activity was absent and BA did not induce the activity. Another characteristic of the monooxygenase enzyme system is its ability to activate chemically inert compounds with the formation of toxic metabolites. As shown in Fig. 1, BP has pronounced toxicity in the hepatoma McA RH 7777 culture, whereas the number of hepatoma 27 cells increased versus control after BP administration. These data confirm the lack of constitutive and induced cytochrome P450-dependent activities in the hepatoma 27 cells. The constitutive activity of enzymes of metabolic phase II was determined by the activity of NADP(H)-quinone oxidoreductase; this activity was detected both in hepatoma 27 and in hepatoma McA RH 7777. Like the monooxygenase activity, induction of NADP(H)-quinone oxidoreductase by bifunctional inducers BA and beta-NF was detected only in the hepatoma McA RH 7777 cells (Fig. 2a). The monofunctional inducers PCMB and dimethylfumarate induced NADP(H)-quinone oxidoreductase activity in hepatoma McA RH 7777 but not in hepatoma 27 (Fig. 2b).
Table 1. Effect of benz[a]anthracene (BA) on
the activity of benzo[a]pyrene hydroxylase (BPH) and 7-ethoxyresorufin
deethylase (7-EROD) (pmole/min per 106 cells) in hepatoma
McA RH 7777 and hepatoma 27
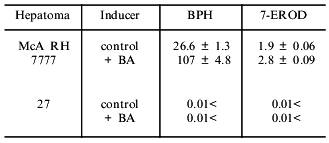
Fig. 1. Toxicity of benzo[a]pyrene (BP) in cultures of hepatoma 27 (1) and hepatoma McA RH 7777 (2) as determined by the MTT test.
Thus, in hepatoma 27 cells neither pathway for transduction of enzyme-inducing signal is functional.Fig. 2. Changes in the activity of NADP(H)-quinone oxidoreductase in hepatoma 27 and hepatoma McA RH 7777 cells in the presence of: a) bifunctional inducers; b) monofunctional inducers. C, control; BA, benz[a]anthracene (5 µg/ml); beta-NF, beta-naphthoflavone (1 µM); DMF, dimethylfumarate (40 µM); PCMB, p-chloromercuribenzoate (50 µM).
The proliferative effects of bifunctional inducers on cultured hepatoma 27 and hepatoma McA RH 7777 cells were compared in the second part of the work. Various bifunctional inducers stimulate proliferation of both types of hepatoma cells (Table 2). Phenanthrene (PAH) had no inducing or mitogenic activities.
Table 2. Effect of bifunctional inducers on
the number of mitoses in hepatoma 27 and hepatoma McA RH 7777 cells

PAH-stimulated induction of cytochrome P450 isoforms is known to be PKC-dependent. Suppression of PKC activity by addition of its inhibitors [14] or by desensitization via prolonged incubation of the cells with phorbol ester [15] abolishes the induction. We studied the role of PKC in the proliferative action of PAH. Incubation of hepatoma McA RH 7777 cells with TPA for 72 h (as previously shown [15], this results in exhaustion of PKC in the cell) blocks the induction of 7-EROD caused by BA administration. 7-EROD activity is induced by BA up to 12.5 nmoles/min per 106 cells; in combination with 150 nM TPA, 7-EROD activity decreased to 3.6 nmoles/min per 106 cells. However, co-administration of TPA and BA in the hepatoma 27 cell culture had a weak inhibitory effect on the mitogenic effects of BA (Table 3), thus suggesting that PKC function is not required for the mitogenic effects of PAH.
Table 3. Effect of benz[a]anthracene (BA)
and 12-O-tetradecanoylphorbol 13-acetate (TPA) on the number of mitoses
in a culture of hepatoma 27 cells
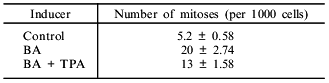
Ah-receptor is the main target of bifunctional inducers in the cells resulting in the synthesis of cytochrome P450 isoforms. alpha-NF is an antagonist of the Ah-receptor. Combined administration of alpha-NF and Ah-receptor agonist suppresses the induction. To elucidate the role of Ah-receptor in the mitogenic effect of PAH, we studied the influence of alpha-NF on proliferative potential of the cultured hepatoma 27 cells. In hepatoma McA RH 7777 cells, alpha-NF inhibits the induction of BPH by BA (Table 4). Surprisingly, in the hepatoma 27 cells alpha-NF by itself has a pronounced mitogenic effect (Table 5). Co-administration of BA and alpha-NF lowered the level of proliferation compared to separate administration of BA and alpha-NF.
Table 4. Effect of
alpha-naphthoflavone (NF) on 7-ethoxyresorufin deethylase
(7-EROD) activity (pmole/min per 106 cells) in hepatoma McA
RH 7777 cells

Table 5. Effect of benzo[a]pyrene (BP) and
alpha-naphthoflavone (alpha-NF) on the number of mitoses
in hepatoma 27 cells

DISCUSSION
Synthesis of new isoforms of cytochrome P450 is the final stage of events triggered in the cell by inducers. Lack of cytochrome P450 in the hepatoma 27 cells is not associated with mutations of the signal-transducing proteins and genes of cytochrome P450 isoforms because the very same hepatoma 27 cells transplanted into liver can be induced by PAH. Lack of induction of NADP(H)-quinone oxidoreductase in this culture is not associated with mutations of the gene of this protein because the constitutive level of enzyme activity is expressed. Bifunctional inducers do not induce the enzyme but still stimulate proliferation of the hepatoma 27 cells. Thus, the pathways of mitogenic and inducing signal transduction are either completely different or they diverge at some stage.
Bifunctional inducers activate the genes involved in mitogenic signal transduction as soon as 4 h after their administration to the cell culture, i.e., before new cytochrome P450 isoforms are synthesized [8].
A possible mechanism for the proliferative activity of the inducers might be associated with the formation of a complex with the Ah-receptor. This interaction would result in the release of protein kinases which are inactive in the complex with the Ah-receptor [16]. Mitogenic effects of PAH apparently involves Ah-receptor because phenanthrene (which is not a ligand of the Ah-receptor) does not stimulate proliferation of the hepatoma 27 cells. The mitogenic effect occurs prior to phosphorylation of the ligand--receptor complex by PKC because when the enzyme is exhausted with TPA, BA or BP can still stimulate proliferation in the hepatoma 27 cells.
However, our data on alpha-NF-dependent stimulation of proliferation of hepatoma 27 cells are in contradiction with a suggestion that the previously-described immediate response gene activation by ligands of Ah-receptor is the proliferation-triggering mechanism. It was demonstrated that alpha-NF prevents activation of the early response genes by ligands of the Ah-receptor [8]. Also, alpha-naphthoflavone can increase intracellular Ca2+ concentration in mammary gland, indicating that the Ca2+-dependent pathway of mitogenic signal transduction is activated [17]. Antagonistic activity of alpha-NF is due to its interaction with certain regions of the Ah-receptor. The complex blocks the interaction of an agonist with the binding site and suppresses dissociation of heat shock proteins that maintain the Ah-receptor in an inactive form [18].
The interaction of alpha-NF with the Ah-receptor may activate transduction of mitogenic signal via alternative routes which are not associated with the pathways involved in the effects of Ah-receptor agonists.
The work was supported by the Russian Foundation for Basic Research (grant No. 98-04-49091).
REFERENCES
1.Prochaska, H., and Talalay, P. (1988) Cancer
Res., 48, 4776-4782.
2.Nebert, D., Puga, A., and Vasiliou, V. (1993)
Ann. N. Y. Acad. Sci., 685, 624-640.
3.Wasserman, W., and Fahl, W. (1997) Proc. Natl.
Acad. Sci. USA, 94, 5361-5366.
4.Nguyen, T., Rushmore, T., and Pickett, C. (1994)
J. Biol. Chem., 269, 13656-13662.
5.Buchmann, A., Stinchcombe, S., Korner, W.,
Hagenmaier, H., and Bock, K. (1995) Carcinogenesis, 16,
1143-1150.
6.Schulte-Hermann, R., Ohde, G., Schuppler, J., and
Timmermann-Trosiener, I. (1981) Cancer Res., 41,
2556-2562.
7.Koblyakov, V. A. (1998) Biochemistry
(Moscow), 63, 885-898.
8.Puga, A., Nebert, D., and Carrier, F. (1992) DNA
Cell Biol., 11, 269-281.
9.Kobliakov, V., Kulikova, L., Kolyada, A., Chemeriz,
G., and Turusov, V. (1993) Xenobiotica, 23, 703-708.
10.Dohnen, W., Tomluger, R., and Roos, J. (1973)
Anal. Biochem., 33, 373-383.
11.Wortelboer, H., and De Kruif, C. (1990)
Biochem. Pharmacol., 40, 2525-2534.
12.Carmichael, J., De Graff, W., and Gazdar, A.
(1987) Cancer Res., 40, 936-942.
13.Prochaska, H., and Talalay, P. (1986) J. Biol.
Chem., 261, 1372-1378.
14.Chun, Y., Koh, W., and Yang, K. (1994)
Biochem. Mol. Biol. Int., 32, 1023-1032.
15.Okino, S., Pendurthi, U., and Tukey, R. (1992)
J. Biol. Chem., 267, 6991-6998.
16.Enan, E., and Matsumura, F. (1995) Biochem.
Pharmacol., 49, 249-261.
17.Tannheimer, S., Barton, S., Ethier, S., and
Burchiel, S. (1997) Carcinogenesis, 18, 1177-1182.
18.Gasiewicz, T., and Rucci, G. (1991) Mol.
Pharmacol., 40, 607-612.