Comparative Characteristics of Sarcoplasmic Reticulum Preparations from Skeletal Muscles of the Ground Squirrel Spermophilus undulatus, Rats, and Rabbits
A. N. Shutova1, K. B. Storey2, O. D. Lopina1, and A. M. Rubtsov1*
1Department of Biochemistry, School of Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (7-095) 939-3955; E-mail: amr@atpase.bio.msu.su2Department of Biology and Department of Chemistry, Carleton University, Ottawa K1S 5B6, Canada; fax: (613) 520-2569; E-mail: kbstorey@ccs.carleton.ca
* To whom correspondence should be addressed.
Received May 21, 1999; Revision received June 21, 1999
A comparison of sarcoplasmic reticulum (SR) preparations from skeletal muscles of ground squirrels Spermophilus undulatus, rats, and rabbits established that on the basis of protein yield and phospholipid/protein ratio these preparations are practically the same. Nevertheless, the specific activity of Ca-ATPase, the main protein component of SR membranes, in SR preparations of the ground squirrel skeletal muscles is only about half of the activity in SR preparations of rats and rabbits. Significant differences in protein composition of the preparations were detected: ground squirrel SR differed by an unusually high content of a 205 kD protein (probably myosin) and a number of low-molecular-weight SR protein components, and the SR preparations of rabbits are characterized by a high content of the Ca-binding proteins calsequestrin and sarcalumenin. Use of the anionic carbocyanine dye Stains-All established that all preparations contained only three proteins which are stained dark blue by this dye: calsequestrin, sarcalumenin, and a histidine-rich Ca-binding protein. The electrophoretic mobility of calsequestrin was identical in all preparations (molecular mass 63 kD), whereas sarcalumenin and histidine-rich Ca-binding protein are probably present in different isoforms with molecular masses of 130, 145, and 160 and 165, 155, and 170 kD, respectively, in SR preparations of ground squirrels, rats, and rabbits. Analysis of the fluorescence parameters of the fluorescent probes 8-anilino-1-naphthalene sulfonic acid and pyrene bound to SR membranes showed that the properties of the lipid bilayer in the SR membranes of the preparations differed considerably. It is suggested that the differences in protein composition and/or structural state of the ground squirrel SR membrane lipid bilayer could be the reason for the low Ca-ATPase activity in these preparations.
KEY WORDS: sarcoplasmic reticulum, Ca-ATPase, Ca-binding proteins, calsequestrin, sarcalumenin, histidine-rich Ca-binding protein, ground squirrel Spermophilus undulatus
Sarcoplasmic reticulum (SR), a highly developed intracellular membrane network, plays a key role in excitation--contraction coupling in different types of muscles. Ca-release channels (ryanodine receptors) located in the junction facing membranes of SR terminal cisterns in close proximity to T-tubules provide fast efflux from the SR lumen of the Ca2+ necessary for muscle contraction, whereas Ca-ATPase located in SR longitudinal tubules removes Ca2+ from the cytoplasm using the energy of ATP hydrolysis and leads to the relaxation of muscles [1, 2]. In skeletal muscles the signal transduction from sarcolemma to SR is provided by a direct mechanical contact of plasma membrane L-type Ca-channels (dihydropyridine receptors) and SR Ca-release channels [3]. A number of SR proteins--triadin, calreticulin, calsequestrin, sarcalumenin, histidine-rich Ca-binding protein, calmodulin, and some others--are involved in regulation of the interaction of plasma membrane L-type Ca-channels and SR Ca-release channels [4, 5]. Therefore, the normal functioning of SR Ca-release channels is controlled by protein--protein interactions. Probably, protein--protein interactions play a particular role in the regulation of SR Ca-ATPase activity: in cardiac SR phospholamban is an endogenous regulator of Ca-ATPase [6], and in skeletal muscle SR calsequestrin, calreticulin, sarcalumenin, and 53-kD glycoprotein are putative regulators of Ca-ATPase [7-9].
In contrast with the regulation of excitation--contraction coupling, an additional function that can be provided by skeletal muscle SR is less well understood. This is the participation of SR Ca-release channels and Ca-ATPase in so-called “nonshivering thermogenesis” [10]. In fact, if acting simultaneously, Ca-release channels which provide Ca2+ release from SR lumen into the cytoplasm and Ca-ATPase which removes Ca2+ from the cytoplasm back into the SR lumen form a classical futile cycle, the only net result of which is ATP hydrolysis and heat production. This function of SR is well expressed in the so-called “heating organ” of some fish species of the Xiphiidae, Istiophoridae, and Scombridae families [11]. The modified muscle cells of this organ largely lack the proteins of the contractile apparatus, whereas the network of SR membranes in these cells is very well developed. Futile cycling via Ca-release channels--Ca-ATPase in these cells provides the heat production that is necessary for a maintaining high temperature in the eyes and brain of these fishes. In addition, the activation of the Ca-release channels--Ca-ATPase futile cycle in skeletal muscles results in so-called “tuna burn” which occurs during fishing for some tuna species [11]. This phenomenon is connected with a sharp increase in the temperature of the fish skeletal muscles that causes death and the loss of selling quality. It should be noted that this phenomenon seen in fish skeletal muscles is practically identical with the changes that occur in skeletal muscles of pigs that are susceptible to so-called “malignant hyperthermia”. In the skeletal muscles of these pigs, a mutant form of the SR Ca-release channel is present which is activated under stress conditions (in particular, at the slaughterhouse). This leads to the activation of the Ca-release channels--Ca-ATPase futile cycle and to the death of the pigs as a result of heat shock [10, 11].
The role of SR Ca-release channels and Ca-ATPase in mammalian muscle thermogenesis has not yet been very well studied. However, there is some evidence that the SR can play a major role in skeletal muscle nonshivering thermogenesis during the awakening of hibernating mammals [10, 12]. Brown fat is very well developed in hibernators and provides heating mainly to brain and heart but ~70% of the heat production during awakening is actually in skeletal muscles [12, 13]. During awakening, the electrical activity of skeletal muscle motor nerves is sharply increased even at very low body temperature, whereas muscle contraction does not occur until body temperature has increased up to 12-14°C [13]. Because the stimulation of motor nerves leads to the activation of SR Ca-release channels [3-5], it may be that at low body temperature heat production in muscle is occurring via nonshivering thermogenesis due to the SR Ca-release channels--Ca-ATPase futile cycle. When body temperature rises to higher values this would then be replaced (or supported) by shivering thermogenesis involving the proteins of the contractile apparatus.
Until now the peculiarities of the organization and functional activity of skeletal muscle SR in hibernating animals were largely unknown. To explore these, the main goal of the present study was to determine the general characteristics of SR preparations from skeletal muscles of a typical hibernator, the ground squirrel Spermophilus undulatus, in comparison with SR preparations from skeletal muscles of rats and rabbits.
MATERIALS AND METHODS
Materials. Fluorescent probes (8-anilino-1-naphthalene sulfonic acid (ANS) and pyrene), ATP, ADP, Mops, NADH, sodium deoxycholate, histidine, imidazole, sucrose, glycine, EGTA, and EDTA were purchased from Sigma (USA). Tris, pyruvate kinase, and phosphoenolpyruvate (PEP) were from Reanal (Hungary); lactate dehydrogenase was from Ferak (Germany); SDS and the carbocyanine dye Stains-All were from Serva (Germany). All other reagents were “reagent grade” or better.
Animals. Adult ground squirrels Spermophilus undulatus were collected by live trapping in Yakutia and were maintained in the Animal Facility of the Institute of Cell Biophysics (Pushchino, Moscow Region) in individual cages at 20-25°C, in natural daylight. The animals were supplied with satisfactory food, water, and nest material. For experiments, active normothermic animals (body temperature 37°C) were killed in June-July by decapitation. Hind leg skeletal muscles were immediately cut off and plunged into liquid nitrogen for transportation. For long storage (for 2-4 weeks), tissues were transferred into a deep freezer (below -70°C). In control experiments, skeletal muscles from rat and rabbit were similarly frozen and kept for 1-4 weeks at -70°C. Comparative analysis of SR preparations obtained from frozen and fresh muscles has shown that this treatment does not affect the general SR parameters analyzed in the present study.
Preparations and assays. SR fragments from skeletal muscles of ground squirrels, rats, and rabbits were obtained by differential centrifugation [14] with some modification. In brief, the tissue (10-15 g) was thawed in ice-cold physiological solution, cut into small pieces with scissors, and homogenized using a Waring blender (3 times for 1 min with 1 min breaks) in 4 volumes of solution containing 0.3 M sucrose, 100 µM PMSF, and 10 mM histidine, pH 7.7, at 4°C. The homogenate was centrifuged for 20 min at 12,000g. The supernatant was passed through four layers of gauze and centrifuged for 100 min at 30,000g. The pellet was resuspended using a Potter homogenizer (glass--teflon) at 150 W (3 times for 1 min with 1 min breaks) in 30 ml of solution containing 0.6 M KCl, 0.6 mg/ml BSA, 100 µM PMSF, and 5 mM histidine, pH 7.4, held at 4°C for 60 min with stirring, and afterwards centrifuged for 100 min at 30,000g. The final pellet was suspended in 0.25 M sucrose and 25 mM imidazole, pH 7.0, frozen in liquid nitrogen, and stored at -70°C.
Protein concentration was measured according to Lowry et al. [15] using BSA as a standard. The content of phospholipids in SR preparations was measured after mineralization followed by Pi measurement according to Bartlett [16].
Ca-ATPase activity was measured using a coupled enzyme system (pyruvate kinase + lactate dehydrogenase) on a Hitachi-200-20 spectrophotometer (Japan) at 340 nm. Assays were run at 37°C in medium containing 80 mM KCl, 50 mM Mops, pH 7.0, 5 mM MgCl2, 1 mM EGTA, 2 mM PEP, 2.5 mM ATP, 0.3 mg/ml NADH, 5 units pyruvate kinase, and 10 units lactate dehydrogenase (1 enzyme unit converts 1 µmole/min of substrate at 37°C) [17]. The reaction was started by an addition of 10 µg of SR protein into 1 ml of reaction medium to record Mg-dependent ATP hydrolysis. Next, Ca2+ was added to final concentration 1 mM, and then 2 µl of Ca-ionophore A23187 (1 mg/ml) were added to eliminate the Ca-ATPase inhibition by intravesicular Ca2+.
SDS-PAGE was carried out according to Laemmli [18] using 3% stacking and 3-20% gradient running gels [19]. For electrophoresis 30 µg of SR in sample buffer was loaded in each well. After electrophoresis the gels were fixed and washed 3 times in 25% ethanol and stained with the cationic carbocyanine dye, Stains-All, in a solution containing 25% ethanol, 7.5% formamide, 0.0025% Stains-All, and 30 mM Tris, pH 8.8, for 36-48 h in the dark. The gels were briefly destained in 25% ethanol in the dark and scanned on an UltroScan XL laser densitometer at 595 nm (LKB, Sweden). The gels were finally destained in 25% ethanol in the light. Subsequently, the gels were stained with Coomassie Brilliant Blue R-250 and destained by a standard procedure. Then they were scanned on an UltroScan XL laser densitometer at 595 nm. The protein peak areas were calculated using the GelScanXL program (LKB, Sweden).
In the present study ANS and pyrene were used as the fluorescent probes. ANS (5-100 µM) was added to SR membranes (0.2 mg/ml) in 10 mM Mops, pH 7.0. Sample fluorescence was excited at 360 nm and registered at 480 nm with the spectral width of the exciting and analyzing monochromators slit set for 5-nm resolution. Apparent Kd values for ANS were calculated using double reciprocal (1/F versus 1/[ANS]) plots. Pyrene fluorescence was excited at 335 nm (total fluorescence) and at 285 nm (induced fluorescence) with the spectral width of the exciting monochromator slit set for 5-nm resolution and recorded at 350-470 nm with the spectral width of the analyzing monochromator slit set for 1.5-nm resolution. The medium contained 10 mM Mops, pH 7.0, 100 mM KCl, 0.5 mg/ml SR protein, and 3-12 µM pyrene. The extent of pyrene excimerization was calculated as the ratio of fluorescence intensity at 465 nm (excimeric form) and 373 nm (monomeric form) at pyrene concentration 12 µM. All calculations were carried out using standard methods [20].
Calculations. Each parameter for every individual SR preparation was measured at least 3 times and the mean value for this parameter was calculated for further statistical calculations. In the tables, the mean values for 3-4 individual SR preparations are presented ± standard deviation (mean ± SD). Statistical calculations were made using Student's t-criterion [21].
RESULTS AND DISCUSSION
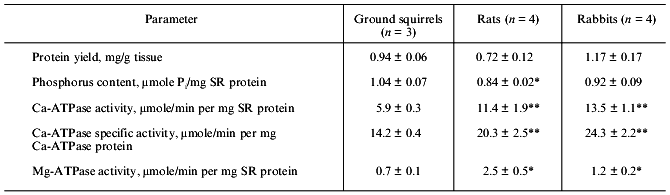
The differential centrifugation method which was used in the present study produces a so-called “total” SR fraction containing both the fragments of SR longitudinal tubules enriched with Ca-ATPase protein and the fragments of SR terminal cisterns enriched with Ca-binding proteins and Ca-release channels. The yield of SR protein from skeletal muscle of ground squirrels, rats, and rabbits was very similar, ~1 mg per g wet tissue. The content of lipid phosphorus measured after mineralization of the samples was slightly higher in the SR of the ground squirrels than that of rats and rabbits (Table 1) and was 0.8-1.1 µmoles Pi per mg protein or 0.5-0.8 mg lipid per mg protein, in full accordance with data in the literature [22].
Table 1. Yield of protein, content of
phospholipid phosphorus, and activity of Ca-ATPase and Mg-ATPase in SR
preparations of ground squirrels, rats, and rabbits
Note: The number of SR preparations used for analysis is shown in
parenthesis. Asterisks indicate values that are significantly different
from the corresponding value for SR preparations of ground
squirrels.
*p < 0.05.
**p < 0.01.
Activity of Ca-ATPase, the main protein component of SR membranes, was ~2-fold lower in SR preparations of the ground squirrels than that in SR preparations of rats and rabbits, and activity of Ca-independent Mg-ATPase was equal to ~9, ~12, and ~20% of total ATPase activity in SR preparations of rabbits, ground squirrels, and rats, respectively (Table 1). Because Mg-ATPase is located mainly in the membranes of T-tubules [23], it could be concluded that the preparations used contained different amount of the fragments of these tubules, or that the contents of Mg-ATPase protein in the membranes of T-tubules are significantly different in skeletal muscles of these animals.
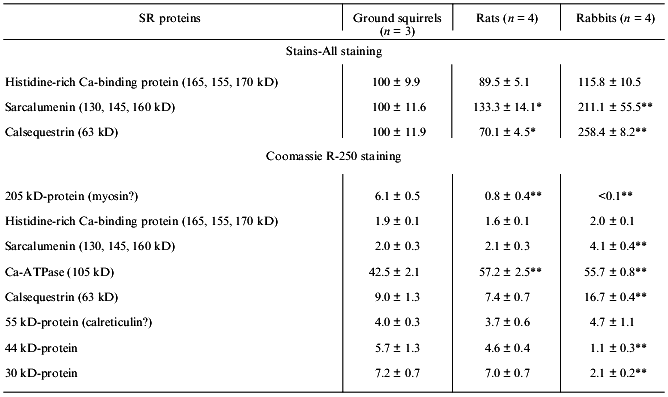
The low activity of Ca-ATPase in the ground squirrel SR preparations might be due to a low content of enzyme protein in SR membranes. To check this possibility, an analysis of SR membrane protein composition of different preparations was carried out using SDS-PAGE. In fact, the content of Ca-ATPase was 40-45% of the total protein in SR preparations of the ground squirrels and 55-60% in SR preparations of rats and rabbits (Table 2). Nevertheless, enzyme specific activity calculated with a correction for its real protein content was still significantly lower in SR preparations of the ground squirrels (Table 1).
Table 2. Contents of the main protein
components in SR membranes of the ground squirrels, rats, and
rabbits
Note: Data obtained from scanning gels stained by the carbocyanine
dye Stains-All are expressed relative to the mean intensity for the
peak area of each protein in SR preparations of ground squirrels. Data
obtained after staining the gels with Coomassie R-250 are expressed as
percentages of the total SR protein peak areas. The number of SR
preparations used for analysis is shown in parenthesis. Asterisks
indicate values that are significantly different from the corresponding
value for SR preparations of ground squirrels.
*p < 0.05.
**p < 0.01.
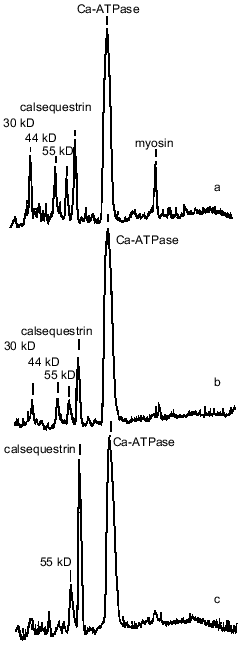
SR preparations from the ground squirrels also significantly differed in the content of some other protein components as compared with rats and rabbits (Fig. 1, Table 2). An unusually high content of a protein with molecular mass 205 kD was observed in SR preparations from ground squirrels; the electrophoretic mobility of this protein was identical to the mobility of myosin heavy chains. This protein band was practically absent in SR preparations of rats and rabbits. Because SR membranes in skeletal muscles are in close contact with the proteins of the contractile apparatus [24], it may be that in ground squirrel skeletal muscles this interaction is so strong that even the treatment of membranes with 0.6 M KCl did not totally remove myosin from the SR preparations. The SR preparations of ground squirrels and rats showed a very similar protein pattern, whereas the SR preparations of rabbits differed considerably, having a higher content of calsequestrin, sarcalumenin, and histidine-rich Ca-binding protein and a lower content of unidentified proteins with molecular masses 44 and 30 kD.
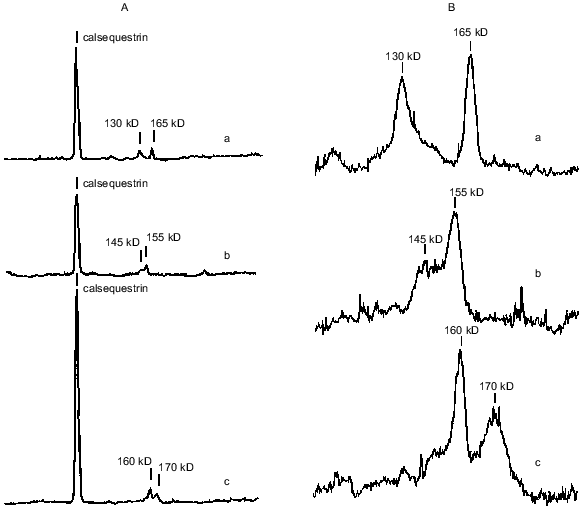
Calsequestrin, sarcalumenin, and histidine-rich Ca-binding protein in SR preparations were identified using the carbocyanine anionic dye (Stains-All) because in SR membranes only these three proteins stain to a dark blue color with this dye [25-27]. All of these are glycoproteins, are located inside the SR network, can bind Ca2+ with low affinity (Kd ~ 1 mM), and, probably, play roles in the SR lumen of Ca-buffers that decrease the concentration of ionized Ca2+. In addition, sarcalumenin and histidine-rich Ca-binding protein are phosphorylated by endogenous SR protein kinases and participate in the regulation of SR Ca-release channels [26, 27]. As seen from Fig. 2A, only three blue-colored protein bands were detected on Stains-All-stained electrophoregrams of SR preparations of the ground squirrels, rats, and rabbits. The content of calsequestrin, with molecular mass 63 kD, was slightly lower in SR of rats and 2-2.5-fold higher in SR of rabbits than in SR of ground squirrels. The two high-molecular-weight proteins, sarcalumenin and histidine-rich Ca-binding protein, showed different electrophoretic mobilities in each of the SR preparations. This suggests that different isoforms of sarcalumenin and histidine-rich Ca-binding protein are present in the SR of these animals: their molecular masses were 130, 145, and 160 kD and 165, 155, and 170 kD in SR preparations of the ground squirrels, rats, and rabbits, respectively (Fig. 2B). The content of histidine-rich Ca-binding protein was approximately the same in SR preparations of all the animals, whereas the content of sarcalumenin was slightly higher in the SR of rats and significantly higher in the SR of rabbits in comparison with the SR of the ground squirrels (Table 2).Fig. 1. Densitograms of SR preparations from skeletal muscle of ground squirrels (a), rats (b), and rabbits (c) separated by SDS-PAGE and stained by Coomassie R-250. In each case 30 µg of SR protein was loaded.
Another Ca-binding protein (calreticulin, with molecular mass 55 kD [8, 28]) stains a red color with Stains-All. A protein with a molecular mass of 55 kD was present on electrophoregrams of all SR preparations; its content was measured after staining the gels with Coomassie R-250 and was approximately the same in all preparations (Table 2). This suggests that the content of calreticulin was the same in the SR of all three species; however, it is also possible that the 55-kD protein band contained other proteins with similar electrophoretic mobility.Fig. 2. A) Densitograms of SR preparations from skeletal muscle of ground squirrels (a), rats (b), and rabbits (c) separated by SDS-PAGE and stained with the carbocyanine dye Stains-All. B) An enhanced view of the region containing proteins of 100-200 kD. In each case 30 µg of SR protein was loaded.
Because Ca-ATPase activity strongly depends on the phospholipid composition and structural state of the SR membrane lipid phase [29, 30], the properties of the SR membrane surface and hydrophobic core were analyzed with the use of the fluorescent probes ANS and pyrene, respectively. The quantum yield of ANS fluorescence in aqueous solution is extremely low, but after ANS binding to the membrane surface (which is controlled by both hydrophobic and electrostatic interactions) ANS fluorescence increased sharply as a result of fluorophore transition into a more hydrophobic and rigid environment [20, 31]. As a result of this, the maximum level of ANS fluorescence depends on both the number of binding sites on the membrane and the properties of its microenvironment. As seen from Table 3, SR preparations from ground squirrels had the highest affinity for ANS among the three species, but the maximum ANS binding (maximal fluorescence) was ~1.5- and ~3-fold higher in rats and rabbits, respectively, than in ground squirrel SR preparations. Therefore, it can be suggested that the number of ANS binding sites on SR membranes of the ground squirrels is significantly lower than that on SR membranes of rats and rabbits and/or that ANS bound to ground squirrel SR membranes is located in more hydrophilic or less rigid microenvironment than in the other species.
Table 3. Parameters of ANS and pyrene
fluorescence in SR membranes of ground squirrels, rats, and rabbits
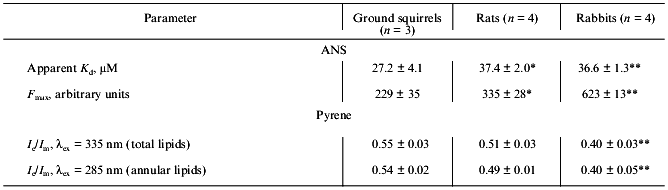
Note: The number of SR preparations used for analysis is shown in
parenthesis. Asterisks indicate values that are significantly different
from the corresponding value for SR preparations of ground
squirrels.
*p < 0.05.
**p < 0.01.
The hydrophobic fluorescent probe (pyrene) penetrates into the inner core of the SR lipid bilayer where the hydrocarbon chains are located. This probe can form excimers--dimers consisting of one excited and one unexcited pyrene molecule with their fluorescence maximum shifted into a higher-wavelength region in comparison with the fluorescence maximum of monomers. The degree of pyrene excimerization (the ratio of fluorescence intensity of excimers and monomers of pyrene) is a diffusion-controlled process and depends on membrane hydrophobic volume and its microviscosity [20, 31]. In addition, it is also possible to induce fluorescence of some of the probe molecules which are located in close proximity to the Ca-ATPase hydrophobic domain (within the Forster radius) due to resonance energy transfer from tryptophan to pyrene after excitation of Ca-ATPase tryptophan residues. This “induced” pyrene fluorescence characterizes the structural state of annular lipids tightly bound to the Ca-ATPase hydrophobic domain in the SR membrane (~30 moles of phospholipids per mole of enzyme) [20, 31].
As seen from Table 3, the degree of pyrene excimerization was greatest in SR membranes of ground squirrels and lowest in SR membranes of rabbits. Because the phospholipid/protein ratio in these preparations is virtually the same (Table 1), we suggest that the differences in degree of pyrene excimerization are not due to differences in SR membrane hydrophobic volume, but to a lower microviscosity of the membrane lipid bilayer in SR of ground squirrels. This conclusion is supported by the very similar values for the degree of pyrene excimerization for annular lipids in all preparations studied (Table 3). In fact, during the summer months the ground squirrels accumulate significant amounts of polyunsaturated fatty acids in different tissues that are necessary for survival during winter hibernation [12, 32]. A high content of polyunsaturated fatty acids is probably the main reason for a low microviscosity of the hydrophobic zone of SR membranes in ground squirrels.
Therefore, our data show that SR preparations from skeletal muscles of two representatives of the order Rodentia, the ground squirrel S. undulatus and rat, have the same protein composition (Table 2, Fig. 1) but differ strongly in the properties of their lipid bilayers (Table 3) and in their Ca-ATPase activity (Table 1). Preparations of SR from rabbit skeletal muscles (order Lagomorpha) differ from both of the rodents in protein composition and in the properties of the lipid bilayer. However, the specific activity of Ca-ATPase in SR preparations of rats and rabbits was virtually identical. The data suggest that the low Ca-ATPase specific activity in SR preparations of ground squirrels is connected with the species-specific peculiarities of the isoform expressed in skeletal muscles of these hibernators. However, the possibility also exists that the properties of the lipid bilayer and/or minor protein components of the SR membranes may specifically affect Ca-ATPase activity.
The three SR preparations also differed in their contents of low-affinity Ca-binding proteins (Table 2, Fig. 2A). In addition, sarcalumenin and histidine-rich Ca-binding protein appeared to be present in species-specific isoforms in skeletal muscle SR, each protein showing a different electrophoretic mobility in each of the three species (Fig. 2B). Because these proteins are glycoproteins the differences in their electrophoretic mobility might be connected with a different composition of their carbohydrate components, but differences in their protein component are also possible. It should be noted that these proteins are directly involved in the regulation of SR Ca-release channel activity: phosphorylation of sarcalumenin and histidine-rich Ca-binding protein by endogenous protein kinases inhibits Ca-release channels [25, 26]. Calsequestrin and calsequestrin-binding protein with molecular mass 30 kD are also involved in the regulation of SR Ca-release channels [7, 9, 33]. Probably the 30-kD protein which is present in SR preparations of ground squirrels and rats (Table 2) is the calsequestrin-binding protein; however, it will be necessary to conduct further studies to confirm its identity.
In conclusion, SR preparations from skeletal muscle of ground squirrels differ from rat and rabbit SR in a number of parameters (Ca-ATPase activity, protein composition, high content of protein with molecular mass 205 kD (myosin), different isoforms of sarcalumenin and histidine-rich Ca-binding protein, physicochemical characteristics of phospholipid bilayer). What is still unknown is whether these differences are related to hibernation, providing necessary adjustments that aid low-temperature function of the SR or contributing to nonshivering thermogenesis during arousal. To answer this question, it will be necessary to investigate the functional activities of SR Ca-ATPase and Ca-release channels in ground squirrel skeletal muscles during winter hibernation. These studies are now in progress in our laboratory.
Authors thank R. Kh. Ziganshin and D. A. Ignat'ev for the kindly provided samples of the ground squirrel tissues. The research described in this publication was made possible in part by grant No. 98-04-49184 from the Russian Foundation for Basic Research and by grant No. OGP 6793 from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
1.Inesi, G. (1985) Ann. Rev. Physiol.,
47, 573-601.
2.Martonosi, A. (1984) Physiol. Rev.,
64, 1240-1320.
3.Sutko, J., and Airey, J. A. (1996) Physiol.
Rev., 76, 1027-1071.
4.Rubtsov, A. M., and Batrukova, M. A. (1997)
Biochemistry (Moscow), 62, 933-945.
5.Wagenknecht, T., and Rademacher, M. (1997) Curr.
Opin. Struct. Biol., 7, 258-265.
6.Simmerman, H. K., and Jones, L. R. (1998)
Physiol. Rev., 78, 921-947.
7.Yano, K., and Zarain-Herzberg, A. (1994) Mol.
Cell. Biochem., 135, 61-70.
8.Krause, K.-H., and Michalak, M. (1997) Cell,
88, 439-443.
9.Froemmig, G. R., and Ohlendieck, K. (1998)
Biochim. Biophys. Acta, 1387, 226-238.
10.Block, B. A. (1994) Ann. Rev. Physiol.,
56, 535-577.
11.Block, B. A., Finnerty, J. R., and Stewart, A. F.
R. (1993) Science, 260, 210-214.
12.Panteleev, P. A. (1983) Bioenergetics of Small
Mammals [in Russian], Nauka, Moscow.
13.Lyman, C. P. (1982) in Hibernation and Torpor
in Mammals and Birds (Lyman, C. P., Willis, J. S., Malan, A., and
Wang, L. C. H., eds.) Academic Press, N. Y., pp. 104-123.
14.Ritov, V. B., Melgunov, V. I., Komarov, P. G.,
Alekseeva, O. M., and Akimova, E. I. (1977) Dokl. Akad. Nauk
SSSR, 233, 727-733.
15.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) J. Biol. Chem.,193, 265-275.
16.Bartlett, G. R. (1959) J. Biol. Chem.,
234, 466-468.
17.Anderson, K. W., and Murphy, A. J. (1983) J.
Biol. Chem.,258, 14276-14278.
18.Laemmli, U. K. (1970) Nature
(London),227, 680-685.
19.Shorina, E. A., Mast, N. V., Lopina, O. D., and
Rubtsov, A. M. (1997) Biochemistry, 36, 13455-13460.
20.Lakowicz, J. R. (1983) Principles of
Fluorescence Spectroscopy, Plenum Press, N. Y.
21.Sepetliev, D. (1968) Statistical Methods in
Scientific Medical Researches [in Russian], Meditsina, Moscow.
22.Tada, M., Yamamoto, T., and Tonomura, Y. (1978)
Physiol. Rev., 58, 1-79.
23.Rosemblatt, M. S., and Scales, D. J. (1989)
Mol. Cell. Biochem., 87, 57-69.
24.Zhou, D., Birkenmeier, C. S., Williams, M. W.,
Sharp, J. J., Baker, J. E., and Bloch, R. J. (1997) J. Cell
Biol., 136, 621-631.
25.Campbell, K. P., MacLennan, D. H., and Jorgensen,
A. O. (1983) J. Biol. Chem., 258, 11267-11273.
26.Orr, I., and Shoshan-Barmatz, V. (1996)
Biochim. Biophys. Acta, 1283, 80-88.
27.Shoshan-Barmatz, V., Orr, I., Well, S., Meyer,
H., Varsanyi, M., and Heilmeyer, L. M. (1996) Biochim. Biophys.
Acta, 1283, 89-100.
28.Coppolino, M. G., and Dedhar, S. (1998) Int.
J. Biochem. Cell Biol., 30, 553-558.
29.Lee, A. G. (1991) Prog. Lipid
Res., 30, 323-348.
30.Lee, A. G., Dalton, K. A., Duggleby, R. C., East,
J. M., and Starling, A. P. (1995) Biosci. Rep.,
15, 289-298.
31.Vladimirov, Yu. A., and Dobretsov, G. E. (1980)
Fluorescent Probes in the Study of Biological Membranes [in
Russian], Nauka, Moscow.
32.Kalabukhov, N. I. (1985) Hibernation of
Mammals [in Russian], Nauka, Moscow.
33.Kagari, T., Yamaguchi, N., and Kasai, M. (1996)
Biochem. Biophys. Res. Commun.,227, 700-706.