Problems and Perspectives in Studying the Biological Role of Carnosine
Introduction of the Guest-Editor
A. A. Boldyrev
International Center for Biotechnology, Department of Biochemistry, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (7-095) 939-1398; E-mail: aab@genebee.msu.su
Received December 17, 1999
In describing carnosine among the constituents of muscle tissue in 1900, V. Gulevitsch opened the question of its real biological role. Investigation of carnosine-related phenomena occurred simultaneously with the study of its metabolic transformation within the cell. It has now been demonstrated that carnosine has the ability to protect cells against oxidative stress as well as to increase their resistance toward functional exhaustion and accumulation of senile features. Mechanisms of such protection are explained in terms of proton buffering, heavy metal chelating, as well as free radical and active sugar molecule scavenging, preventing modification of biomacromolecules and keeping their native functional activity under oxidative stress. Several carnosine derivatives are characterized by different rates of splitting by tissue carnosinase and by different biological efficiencies, thus the biological significance of enzymatic modification of carnosine during its tissue metabolism may be increased resistance of cells operating under unfavorable conditions.
KEY WORDS: carnosine, metabolic transformation, excitable tissues, oxidative stress, mobile proton buffer, cellular antioxidants
Nature, like a Sphinx,
And the temptation of humans with Her mysteries is stronger
Considering that maybe there are no mysteries at all.
F. I. Tyutchev
Carnosine (beta-alanyl-L-histidine) was discovered and its structure determined in the very beginning of the XX century [1, 2]. It was the first and the simplest example of biologically active peptides (actually a dipeptide), opening the long list of widespread natural protein regulators of metabolism. After the first decades dedicated to studies of structure, distribution, and properties of the compound, Gulevitsch clearly understood [3] that carnosine has a direct relation to the function of excitable tissues (at that time, carnosine and related substances had been found only in muscle tissues, whereas these compounds were found later in vertebrate brain, see for references [4]).
The biological role of carnosine is illustrated by a clear correlation between its concentration and the functional activity of muscles, by the period of its accumulation related to a specific step of ontogeny, and by the presence of special enzymes providing synthesis and metabolic transformation of carnosine. These data were accumulated step-by-step by V. Gulevitsch and his students and were systematically presented by S. Severin in his plenary lecture at the VI International Congress on Biochemistry in New York in 1964 [5].
The very first reference to the biological significance of carnosine as an effective biological pH buffer was made by E. Bate-Smith [6]. This suggestion was developed by V. Skulachev [7], who stressed the importance of a mobile proton buffer for those tissues which, like skeletal muscles, have a preferentially glycolytic type of energy supply, and by H. Abe (see [8], as well as his article in this issue), who calculated the buffer capacity provided by carnosine and related compounds in muscle tissue. In addition, the ability of the dipeptide to form complexes with copper, zinc, cobalt, and ferrous ions (see review of E. Baran in this issue) extended its functional role by regulation of the content of these ions in biological fluids and tissues.
Further study of carnosine and its natural derivatives showed that pH buffering in not their only biological role. It was noted repeatedly that the main part of carnosine in muscles is associated with proteins, which hindered its role as a mobile proton buffer. Moreover, several biological functions of carnosine (like support of viability of cultured cells [9]) are not characteristic to its D-isomer, which possesses the same pH-buffering capacity. In 1953, S. Severin and co-workers described restoration of muscle working capacity after addition of carnosine in the medium surrounding a fatigued skeletal muscle. This effect was also characteristic of the N1-methylated derivative, anserine, and because of special experimental precautions, did not depend of their pH-buffering capacity. This phenomenon, later given the name of its demonstrator (Severin's phenomenon), was not mechanistically understood for a long time. Nevertheless, this “mystery” of biological function(s) of carnosine pointed out its specific participation in muscle metabolism.
Several hypotheses were developed to explain this participation. It was suggested that carnosine can serve as a source of histidine and beta-alanine [10], but this suggestion is not valid because carnosine synthase and carnosinase (enzymes providing for formation or degradation of carnosine, i.e., sequestering or releasing its precursors) are located in distinct structures, thus cellular deposits of carnosine are basically not available for hydrolysis [4]. A neuromediatory role of carnosine in specific brain regions was also discussed [11], but specific receptors for this molecule were not found. In such a way, while carnosine is released after depolarization of cultured neuronal cells [12] and co-localization of carnosine with glutamatergic compounds has been demonstrated [13], these data were rather attributed to an ability of carnosine to suppress a toxic component of the neuromediatory function of glutamate [14].
Moreover, since 1964 [5, 15] many facts have been accumulated showing a protecting action of carnosine on a number of enzymes and enzyme complexes, among those such distinct in their mechanism of functioning as dehydrogenases, phosphorylase b, ATPases, and ion pumps (see [4]). A protective effect of carnosine was demonstrated in relation to mitochondrial membranes [15], sarcoplasmic reticulum [16], and (later on) cultured neurons and fibroblasts [14, 17]. It was found that carnosine and related compounds can prevent damage or restore function to damaged biological structures [16].
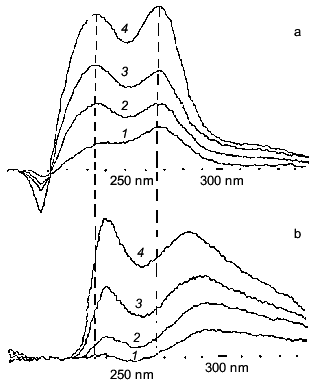
In analyzing the protecting action of carnosine, we will begin from experiments published by E. Neifakh [18]. He noted the lack of correlation between the muscle level of known antioxidants and intensity of induced lipid peroxidation in muscle membranes and suggested that carnosine can provide additional resistance of cells to oxidation by means of restoring the cellular pool of reduced alpha-tocopherol that is converted to oxidized form during protection against radical attack. Later, however, N. Gorbunov and A. Erin demonstrated [19] that carnosine could not reduce the oxidized form of alpha-tocopherol. Moreover, the pool of this antioxidant in muscle is relatively poor. In contrast with this suggestion, a direct interaction of carnosine with molecular products of lipid peroxidation was demonstrated (Fig. 1), as well as with superoxide anion radical (Fig. 2a) and hydroxyl radical (Fig. 2b).
Fig. 1. UV-spectra of primary products of lipid peroxidation accumulated in frog sarcoplasmic reticulum membranes after different periods of incubation: a) in the presence of 25 mM PIPES buffer; b) in the presence of 25 mM carnosine [4]. Incubation time (min): 1) 1; 2) 3; 3) 6; 4) 20.
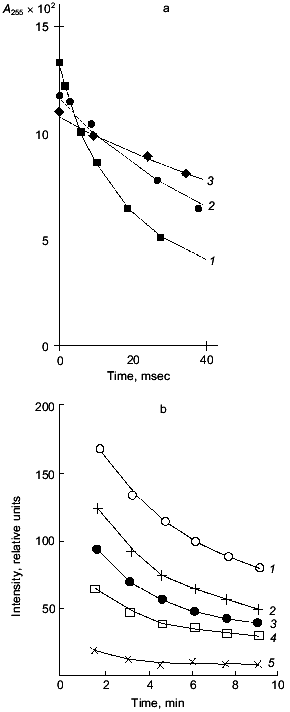
It is necessary to note, returning to Severin's phenomenon, that the main step of electromechanical coupling in fatigued muscle that is affected by carnosine is the sarcoplasmic reticulum Ca-pump, which is inhibited by lipid peroxidation (LPO) and is protected by carnosine or anserine (Fig. 3). Thus, many facts indicate that carnosine works as a hydrophilic antioxidant decreasing the intracellular level of reactive oxygen species [20, 21].Fig. 2. Interaction of reactive oxygen species with carnosine: a) lifetime of oxygen superoxide anion in the absence (1) or presence of 20 µM (2) or 2 mM (3) carnosine (note that while the amount of superoxide anion generated by radiolysis impulse is the same, the initial point of the curves differs depending on the presence and concentration of carnosine and decreases with increase in carnosine concentration); b) decrease in hydroxyl radical concentration in the absence (1) or presence of 1 (2), 2 (3), 5 (4), and 20 mM (5) carnosine. Hydroxyl radical was generated by UV-illumination of H2O2 solution (experimental conditions are described elsewhere [4, 23]).
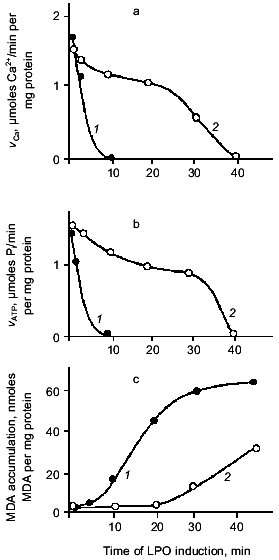
A quantitative description of the interaction of carnosine with the main oxygen radicals is presented in Table 1. It is seen that in the presence of carnosine, the rate of radical neutralization is several times faster. The K0.5 for the antioxidant effect of carnosine is within the range 0.2-10 mM, which is close to its concentrations in muscle and brain (5-40 mM). Actually, carnosine is not as strong a scavenger of free radicals as ascorbate or alpha-tocopherol, but in relation to tissue concentrations of these compounds, it is clear that the antioxidant action of carnosine in excitable tissues has to be very significant [22].Fig. 3. The rate of calcium accumulation (vCa, µmoles Na2+/min per mg protein) (a), Ca-ATPase activity (vATP, µmoles Pi/min per mg protein) (b), and MDA accumulation in membranes of sarcoplasmic reticulum of frog (nmoles MDA per mg protein) (c) after LPO induction by ferrous ions and ascorbic acid in the presence of 25 mM pH buffer PIPES (1) or carnosine (2). For experimental conditions see [20].
Table 1. Interaction of several
antioxidants with reactive oxygen species (ROS)
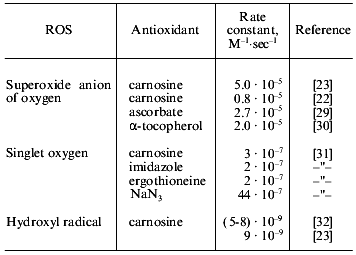
Carnosine was found to form a transfer charge complex with superoxide anion radical, and the toxicity of the radical is suppressed while the lifetime is increased [23]. Such ability results in “buffering” of reactive oxygen species in tissues at a low level, not totally suppressing the cellular function of free radicals as regulatory, signaling, or other important molecules [24]. These facts suggest an especially important role of carnosine for those tissues that use free radicals for regulatory purposes. In brain, for example, the “nitric oxide-superoxide anion” pair regulates blood circulation because NO serves as a vasodilator and O2. - as a vasoconstrictor. With disordered blood supply to the brain (ischemic injury), the appearance of superoxide anion will neutralize the NO effect because of their direct interaction resulting in peroxynitrite formation. Under these conditions, carnosine will both prevent undesirable accumulation of peroxynitrite and demonstrate a positive effect on brain blood supply, this effect being actually demonstrated recently [25].
Comparison of the action of carnosine on the accumulation of LPO products and the activity of the Ca-pump in the same membrane preparations shows an additional protecting effect of the dipeptide (Fig. 3). Inactivation of the Ca-pump by LPO products is noted after 10 min of oxidation, when 20 nmoles of malonic dialdehyde (MDA) per mg protein has accumulated. In the presence of carnosine, the same level of MDA is reached after 30 min, but at this time the Ca-pump is still active (about 40% of control level). These experiments demonstrate that the carnosine effect is not restricted to LPO suppression. The conclusion is that the membrane-protecting effect of carnosine is explained not only by its antioxidant activity [14].
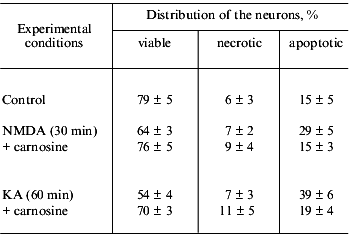
A similar protecting effect of carnosine on cellular structures was also described under osmotic damage to cells, which is not directly related to reactive oxygen species (ROS) [17, 26], as well as during cell culturing [9]. These effects demonstrated an ability of carnosine to prevent toxic action of metabolic products. In all cases, carnosine increased the resistance of cells to unfavorable factors and prevented cellular death--both apoptotic and necrotic (Table 2). At the same time, N-acetylcarnosine, possessing a similar ability to suppress intracellular ROS level, did not increase the viability of neurons [4, 14].
Table 2. Effect of carnosine (1 mM) on
the excitotoxic death of rat cerebellum neurons induced by
N-methyl-D-aspartate (NMDA, 1 mM) or kainate (KA, 1 mM) [33]
All these data were satisfactorily explained by the ability of carnosine to protect cellular structures from aldehydes, which in excess may be toxic because of the non-enzymatic glycosylation of proteins. This process, which is named “glycation”, preferentially modifies the epsilon-amino group of lysine residues in proteins, especially when proline neighbors lysine. It is likely that the structural similarity between lysyl-proline and beta-alanyl-histidine allows carnosine to compete for the glycating agent, protecting proteins against modification [17].
This action may totally protect cellular proteins from aldehydes, and this effect may be the main mechanism by which carnosine protects cells against osmotic shock, oxidative stress, or toxic effects of several metabolites accumulated in the cell culturing medium [4, 17]. Actually, carnosine may prevent accumulation of Amadori products (forming after re-arrangements of products of primary glycation) within cells as well as cross-linking of biomacromolecules [27, 28]. Such an effect appears at high carnosine concentrations (see A. Hipkiss' review in this issue), which is in good correlation with the large amount of carnosine in excitable tissues.
At very high concentrations, carnosine also reverses protein-aldehyde cross-linking, a reaction that is difficult to reverse, thus providing a rejuvenating effect on cultured cells (see review of R. Holliday and G. McFarland in this issue). A recently demonstrated clarifying effect of carnosine on eye lens with senile cataract (which develops as a result of cross-linking of crystallins when antioxidant defense is exhausted) was the very first demonstration of such repairing action of carnosine on animal tissues [21]1. Recently, a similar effect was produced with human beings (see A. Wang et al. in this issue). These facts serve as a prologue to the description of a potent anti-aging effect of carnosine by S. Gallant et al. (this issue) when carnosine is used as a food additive. The effect is not seen with a mixture of histidine and beta-alanine.
1 Editor's note: It was found recently that acetylated (Nalpha) carnosine demonstrates even more potent efficiency in treatment of human senile cataract than carnosine does (M. A. Babizhaev et al. (2000) Biochemistry (Moscow), 65, 588-598; M. Babizhaev, V. Yermakova, A. Deyev, and M.-K. Seguin (2000) J. Anti-Aging Med., 3, 43-62).
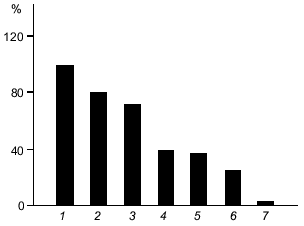
One of the obscure aspects of the carnosine problem is the biological significance of the enzymatic metabolism of carnosine in tissues. One possible explanation relates to the difference in the antioxidant activity of carnosine and its derivatives. Figure 4 presents a comparison of the protecting action of carnosine and related compounds on the oxidation of human serum lipoproteins by hydroxyl radicals. Anserine demonstrates the highest, N-acetylcarnosine the lowest, and carnosine the intermediate activity. Thus, in order to change an antioxidant status, tissue enzymes can modify the carnosine molecule and acetylation will suppress, while methylation will increase the resistance of cells to oxidative stress. Thus, modification of carnosine may serve as a regulator of the intracellular ROS level.
Moreover, transformation of carnosine into its derivatives may change the anti-glycating activity of the molecule. For example, acetylation of carnosine removes its ability to protect neurons against apoptosis [14]. Masking the beta-amino group of the molecule by acetylation results in loss of glycating ability; at the same time, N-acetylcarnosine is similar to carnosine in its antioxidant activity, which depends on the presence of the imidazole ring in its structure. Thus, carnosine and related compounds may act as additional regulators of cell death reactions (see Boldyrev's review in this issue).Fig. 4. Protection by carnosine and related compounds of human serum lipoproteins from Fe-induced oxidation: 1) control; 2) N-acetylcarnosine; 3) N-acetylanserine; 4) homocarnosine; 5) ophidine; 6) carnosine; 7) anserine. All compounds are at 5 mM concentration; control, 5 mM PIPES (pKa = 6.8 is close to that of carnosine). Ordinate, oxidation of lipoproteins (% of control) [4].
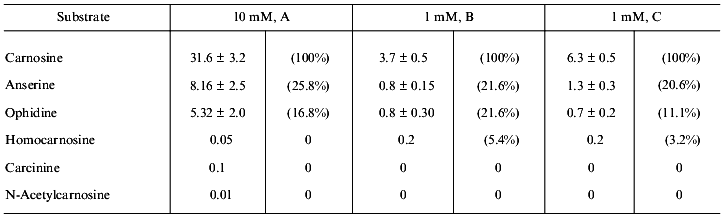
There is the other side of the coin. Neither carnosine nor related compounds are digested by regular peptidases, but they are hydrolyzed by a specific enzyme, carnosinase. Two isoforms of the enzyme are known, localized in serum and kidney; they both have pronounced activity only with carnosine. Anserine and ophidine (N1- and N3-methyl derivatives) are hydrolyzed much more slowly, while homocarnosine, carcinine, and N-acetylcarnosine have negligible rate of hydrolysis (Table 3). Thus, metabolic transformation can protect carnosine against its enzymatic hydrolysis.
Table 3. Hydrolysis of carnosine and related
compounds by kidney carnosinase in the presence (A, B) or absence (C)
of enzyme cofactor (manganese ions) (activity is expressed in nmoles/h
per mg protein or in % to the rate of carnosine hydrolysis (in
brackets)) [34]
In conclusion, modern concepts of the biological role of carnosine and related compounds take into consideration regulation of intracellular concentrations of protons, heavy metals, ROS, as well as active sugars, thus controlling known mechanisms supporting cellular stability. From this point of view, specific localization of these compounds in excitable tissues is fully understandable.
This work summarizes studies of the author and his coworkers at Lomonosov Moscow State University and the Research Institute of Neurology, Russian Academy of Medical Sciences. This work has been supported by the Russian Foundation for Basic Research (grant Nos. 95-04-11969, 96-04-49078, and 99-04-49420) and the Inter-Governmental Russian-Japanese Scientific Program “Conformation of macromolecules and signaling neuropeptides”.
REFERENCES
1.Gulewitsch, W., and Amiradzibi, S. (1900) Ber.
Deutsch. Chem. Ges., 33, 1902-1903.
2.Gulevitsch, W. (1911) Hoppe-Seyler's Z. Physiol.
Chem., 73, 434-443.
3.Gulevitsch, W. S. (1926) Zh. Russ. Fiz.-Khim.
Obsch., 58, 610-616.
4.Boldyrev, A. A. (1998) Carnosine. Biological
Meaning and Possibility of Medical Application [in Russian], Moscow
University Publishing House, Moscow.
5.Severin, S. E. (1964) Proc. Plen. Sess. VI Int.
Congr. Biochem., N. Y., pp. 45-61.
6.Bate-Smith, E. (1938) J. Physiol.,
92, 336-343.
7.Skulachev, V. P. (1989) Energetics of Biological
Membranes, Springer, Berlin.
8.Abe, H. (1995) Biochem. Mol. Biol. Fishes,
4, 310-330.
9.Holliday, R., and McFarland, G. A. (1996) Br. J.
Cancer, 73, 966-971.
10.Aonuma, S., Hama, T., and Tamaki, T. (1970) J.
Biochem., 68, 581-583.
11.Margolis, F. L. (1974) Science,
184, 909-914.
12.Bakarjiev, A. (1998) Glia, 24,
346-351.
13.Berkowitcz, D. A., Trombley, P. Q., and Shepherd,
G. M. (1994) J. Neurophysiol., 71, 2557-2561.
14.Boldyrev, A., Song, R., Lawrence, D., and
Carpenter, D. (1999) Neuroscience, 94, 571-577.
15.Meshkova, N. P. (1964) Uspekhi Biol. Nauk,
6, 86-107.
16.Boldyrev, A. A. (1977) Biochemical Aspects of
Electromechanical Coupling [in Russian], Moscow University
Publishing House, Moscow.
17.Kantha, S., Wada, S., Tanaka, H., Takeushi, M.,
Watabe, S., and Ochi, H. (1966) Biochem. Biophys. Res. Commun.,
223, 278-292.
18.Neifakh, E. A. (1966) Dokl. AN SSSR,
170, 1216-1219.
19.Gorbunov, N. V., and Erin, A. N. (1991) Byull.
Eksp. Biol. Med., 111, 477-478.
20.Severin, S. E., Boldyrev, A. A., and Dupin, A. M.
(1984) Voprosy Med. Khim., 30, 32-36.
21.Boldyrev, A. A., Dupin, A., Bunin, A., Babizhaev,
M. A., and Severin, S. E. (1987) Biochim. Int., 15,
1107-1113.
22.Klebanov, G., Veselkin, Yu., Babenkova, I.,
Popov, I., Levin, G., Tyulina, O., Boldyrev, A., and Vladimirov, Yu.
(1997) Biochem. Mol. Biol. Int., 43, 179-185.
23.Pavlov, A., Revina, A., Dupin, A., Boldyrev, A.,
and Yaropolov, A. (1993) Biochim. Biophys. Acta, 1157,
304-312.
24.Hanchock, J., and Neill, S. (1999)
Biochemist, No. 8, 24-27.
25.Kuklei, M. L., and Gannushkina, I. V. (1997)
Doklady RAN, 352, 416-419.
26.Maltseva, V., Seslavina, L., Matveeva, N., and
Sukhikh, G. (1990) Biol. Nauki, No. 6, 148-153.
27.Hipkiss, A. (1998) Int. J. Biochem. Mol.
Biol., 30, 863-868.
28.Lee, B. J., Park, J. H., Lee, Y. S., Cho, M. H.,
Kim, Y. C., and Hendricks, D. G. (1999) Int. J. Biochem. Mol.
Biol., 32, 370-378.
29.Halliwell, B., and Gutteridge, J. M. C. (1999)
Free Radicals in Biology and Medicine, Oxford University Press,
Oxford-N. Y.
30.Fukuzawa, K., and Gebicki, J. (1983) Arch.
Biochem. Biophys., 226, 242-251.
31.Egorov, S., Kurella, E., Boldyrev, A., and
Krasnovsky, A. (1997) Biochem. Mol. Biol. Int., 41,
687-693.
32.Rao, P. S., Simic, M., and Hayon, E. (1975) J.
Phys. Chem., 79, 1260-1269.
33.Boldyrev, A., Lawrence, D., and Carpenter, D.
(1999) in Peptide Science - Present and Future, Kluwer Academic
Press, Osaka, pp. 413-415.
34.Pegova, A., Abe, H., and Boldyrev, A. (2000)
Comp. Biochem. Physiol., submitted.