REVIEW: Applicability of Zinc Complex of L-Carnosine for Medical Use
T. Matsukura* and H. Tanaka
Research Laboratories, Hamari Chemicals, Ltd. 1-4-29, Kunijima, Higashiyodogawa-ku, Osaka 533-0024, Japan; fax: (81) 6-6326-6744; E-mail: takehumi-matsukura@hamari.co.jp* To whom correspondence should be addressed.
Received November 15, 1999
Zinc complex of L-carnosine (L-CAZ; generic name Polaprezinc) is the first drug for oral administration in which zinc plays an essential role. L-CAZ was approved as an anti-ulcer drug of membrane protection type. Characterization of L-CAZ was achieved by various spectroscopic methods along with elemental analysis. Zinc ion coordinates with L-carnosine to form a quadridentate 1:1 complex of polymeric nature in order to maintain low strain of chelate rings. L-CAZ can remain in stomach juice without rapid dissociation and adhere to ulcerous lesion specifically, after which L-carnosine and zinc are released to heal the ulcer. L-CAZ exhibited high efficacy in clinical use without any serious side effect. L-CAZ exhibited an inhibitory effect on Helicobacter pylori. Physicochemical aspects on carnosine, zinc, and zinc complex can explain favorable features of L-CAZ as a drug.
KEY WORDS: zinc, zinc complex, carnosine, pharmacology, physiology, new anti-ulcer drug
We will present chemical and biological properties of zinc complex of L-carnosine (L-CAZ; generic name Polaprezinc) and the advantageous features of this drug as an anti-ulcer drug of membrane protective type and compare it with other drugs of the same type. In addition, the possibility for the development of new drugs by the combination of zinc, an essential trace metal element of wide significance, with some appropriate ligands, including L-carnosine, will be discussed.
We have developed a unique anti-ulcer drug, composed of L-CAZ [1], and it has been in wide clinical use in Japan since 1994. Studies on L-carnosine have been carried out actively from various points of view for many years, and recent progress of this line of study will be presented in this issue. However, research on metal complexes of L-carnosine has not been actively carried out so far. L-Carnosine is a dipeptide composed of beta-alanine and L-histidine; it forms stable complexes with some metal ions of biological significance, such as zinc and copper.
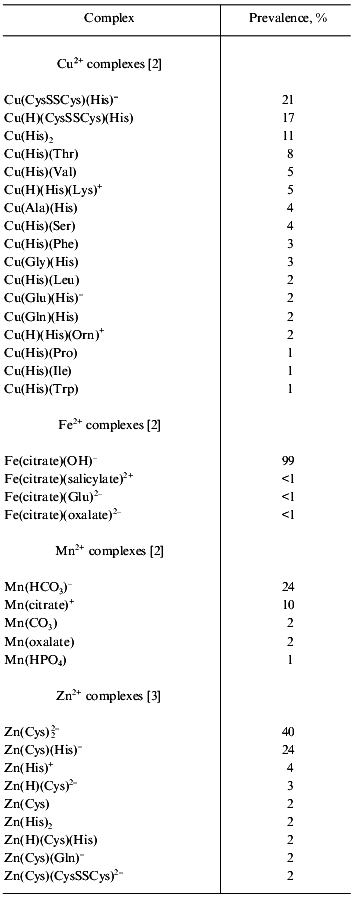
Our aim of the study on the metal complexes of L-carnosine is to investigate the applicability of these complexes for clinical medicine based on the chemical, biological, and pharmacological features of both metal ions and L-carnosine or other ligands. Further, synergistic effect and advantageous properties are expected from complexation. The importance of the presence of a histidine residue in the ligand for complexing with zinc in living systems is clearly shown in Table 1, which shows main chemical forms of zinc present in model human plasma. Accordingly, L-carnosine is regarded as one of the most promising ligands with zinc for application in medicine.
Table 1. Main chemical forms and prevalences
of complexes of trace elements in model human plasma (results
calculated under the conditions: pH 7.4, ionic strength 0.15,
temperature 37°C)
ZINC-L-CARNOSINE (L-CAZ) AS AN ANTI-ULCER DRUG
Discovery. L-Carnosine reacts with zinc acetate in methanol in the presence of sodium methoxide to form white powdery insoluble L-CAZ [1], which presents a sharp X-ray diffraction pattern owing to high crystallinity.
Table 2 shows the healing efficacy of L-CAZ along with those of other zinc complexes against water-immersion stress ulceration in rats. The inhibitory effect was greater than that of cimetidine, which is renowned as an anti gastric ulcer drug all over the world. We have also investigated other metal salts (K, Na, Ca, Mg) of L-carnosine, but no significant effect was observed with those salts or with free L-carnosine. The comparison of the inhibitory effect of L-CAZ, D-CAZ, and cetraxate hydrochloride, which is an approved cytoprotective anti gastric ulcer drug in Japan was also investigated (Table 3). L-CAZ dose-dependently inhibited the formation of gastric ulcers, and the pharmacological activity seems attributable mainly to zinc ion, presumably transported effectively into the ulcer by means of L-carnosine together with the action of L-carnosine itself. Interestingly, D-carnosine with zinc did not show significant anti-ulcer effect, suggesting that non-natural carnosine is not capable of acting as a carrier for the transportation of zinc.
Table 2. Inhibitory effects of zinc
complexes on gastric ulcers induced by water-immersion stress in
rats
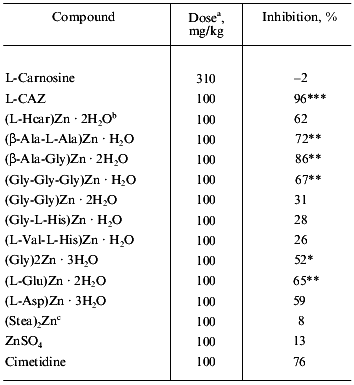
a Oral administration.
b L-Homocarnosine complex.
c Stearic acid salt.
*p < 0.05.
**p < 0.01.
***p < 0.001; here and below the significance of
differences from control was determined by Student's t-test.
Table 3. Inhibitory effects of L-CAZ, D-CAZ,
and cetraxate hydrochloride (CET) on gastric ulcers induced by
HCl-ethanol in rats
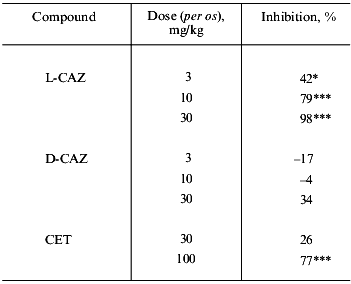
*p < 0.05.
***p < 0.001.
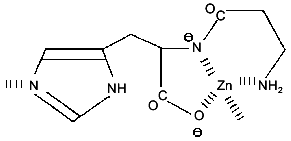
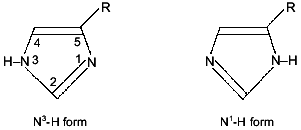
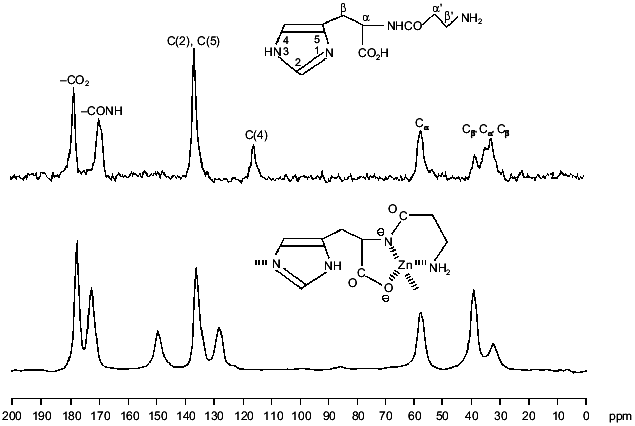
Structure elucidation. Characterization of L-CAZ was achieved by elemental analysis and spectroscopic methods, such as IR, solid-state 13C- and 15N-1H-cross-polarization (CP)/magic angle spinning (MAS) NMR [1]. These data consistently suggested that L-carnosine coordinates to zinc ion as a quadridentate ligand giving a 1:1 complex (Fig. 1). However, the simultaneous binding of those four coordination sites with the same zinc ion as a coordination center should be impossible due to high chelate ring strain, suggesting that L-CAZ is present in polymeric state. Interestingly, the solid state 13C-CP/MAS NMR shows that valence isomerization of the imidazole moiety occurred upon complexation (Fig. 2). The solid state 13C-CP/MAS NMR absorption of the three carbons in the imidazole ring of the histidine moiety was clearly classified in two patterns corresponding to the two valence isomers (Fig. 3). Comparing the absorption patterns of imidazole carbons with those of the related compounds, it was revealed that L-CAZ has a N1-H form, different from the N3-H form of free crystalline carnosine [4]. Such valence tautomerization occurs presumably in order to form energetically favored five- and six-membered chelate rings. The structure of L-CAZ was thus determined definitely as shown in Fig. 1, taking into account thermodynamic stability of the chelate rings. The solid-state 15N-CP/MAS NMR also supported the structure of L-CAZ.
Fig. 1. Structure of L-CAZ.
Fig. 2. Valence tautomers of the imidazole moiety.
Mechanism of action. The slow dissociation rate of L-CAZ in gastric juice due to the polymeric character is essentially important because the prolonged existence in the stomach maintains the healing effect for a long time. Two Japanese research groups [5, 6] found strange specific adherent and penetrative characteristics of L-CAZ to the ulcer lesion by staining zinc with dithizone or Timm solution. We assume that such specific adherence of L-CAZ at the ulcer lesion is attributed to the formation of new chemical bond between zinc and body components, i.e., albumin or other proteins to form mixed ligand complexes. L-Carnosine is considered to still bind with zinc at this stage. These body components oozing from the ulcer site can bind strongly to zinc ion with functional groups such as sulfhydryl or imidazole. Via the stage of the mixed ligand complex, L-carnosine, which has wound healing ability due to the anti-free-radical property [7], is released on the ulcer lesion by complete ligand exchange reaction with a body component capable of forming a complex having a larger stability constant than that of the complex formed from L-carnosine. Simultaneously, zinc, which has a protective effect on membranes, is captured completely by the body component and penetrates into the ulcer to ease inflammation. Such ligand exchange reaction was confirmed by the detection of L-carnosine in supernatant or solution when we mix L-CAZ with various materials such as homogenate taken from stomach of rat, rat serum, albumin, and amino acids with sulfhydryl groups. As the ligand exchange reaction proceeds, insoluble L-CAZ gradually dissolves in water. A simple mixture of L-carnosine and zinc presented less anti-ulceration effect, especially on free-radical induced lesions [8], presumably due to rapid diffusion of L-carnosine and zinc ion in the whole stomach. The stability constant of the complex composed of L-carnosine and zinc [9] is inferred to be suitable for the ligand exchange with body components, giving L-carnosine a characteristic property of an excellent carrier of zinc into the living body. This aspect was also confirmed by a study concerning the absorption of zinc from the intestine, as mentioned below [10].Fig. 3. Solid-state 13C-CP/MAS NMR spectra of L-carnosine and L-CAZ.
Pharmacology. Many pharmacological investigations of L-CAZ in animals have been made, such as wound healing actions [11], membrane stabilizing actions [12], cytoprotection and promotion of mucous defense mechanism [13], and anti-ulcer effects on various types of experimental ulcer models [14-17] including superoxide radical-induced ulcer. From these results, it was concluded that L-CAZ maintains homeostasis of the gastric mucosa by prostaglandin-independent cytoprotective effects due to anti-oxidative membrane stabilizing actions and promotes the repair of damaged tissues by wound healing action.
Clinical studies [18, 19]. Excellent improvement with good compliance was proved in numerous clinical studies by the use of L-CAZ at 150 mg per day administration. Endoscopic healing rates were 27.3 and 64.5% after 4- and 8-week treatments, respectively. The “remarkable improvement” rates of subjective and objective symptoms were 61.3 and 72.0% after 4 and 8 weeks, respectively. Global evaluation of improvement indicated 54.3% “remarkable improvement” and 77.3% including “moderate improvement”. The safety recognition rate was 98.2% and total usefulness rate was 52.9% of “very useful” and 76.5% including “useful”. No symptomatic side effect was reported in 691 cases of clinical trials, indicating the toxicity of L-CAZ should be very low.
Pharmacokinetics. Intestinal absorption of L-CAZ was studied in rats by Sano et al. [20] using 14C- and 65Zn-labeled compounds. They suggested that L-CAZ dissociates to its components, L-carnosine and zinc, during intestinal absorption. They found that the accumulated excretion rates of L-CAZ after a single administration using 14C-labeled L-CAZ to rats are 4.1% in urine, 13.3% in feces, and 38.8% in exhalation, and those using 65Zn-labeled L-CAZ are 0.3% in urine and 85.0% in feces. The absorption rate of zinc was accordingly estimated to be about 11%.
It has been noted that protein-rich diet accelerates zinc absorption [21], which presumably implies that amino acids or peptides with low molecular weight may carry zinc into the blood stream via complexation. A cysteine-rich protein was found in the mucosa of intestine and characterized to be a zinc-carrier protein [22]. In the course of our study as to the healing mechanism of L-CAZ on gastric ulcers, we have been convinced that L-carnosine should act as a good transporter of zinc into the living body.
To evaluate the ability of zinc transportation, various compounds capable of chelation with zinc along with L-carnosine were tested [10]. We employed intraduodenal administration of the mixture of zinc acetate and test ligands in rats. Serum zinc concentrations were determined over seven hours after administration at dose of 10 mg zinc/kg body weight. By comparing the time-dependent serum zinc level with those obtained by intravenous administration (1 mg of zinc acetate), the bioavailabilities of zinc for the test mixtures were calculated.
As shown in Table 4, values of bioavailability of zinc in the presence of L-carnosine, L-homocarnosine, and L-anserine were found to be greater than those obtained with other compounds, except picolinic acid. The optical antipode of L-carnosine did not show any significant effect, which was consistent with the anti-ulcer effect. From these results, it was also confirmed that L-carnosine is a good carrier of zinc into the living body.
Table 4. Bioavailabilities of zinc from
intraduodenal administration of zinc acetate and various compounds
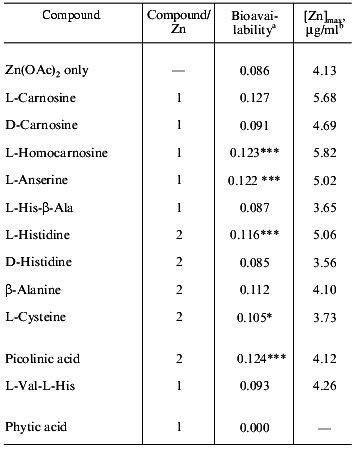
a Bioavailability was determined from the area under the
curve (AUC) of serum zinc concentration versus time after intravenous
(i.v.) and intraduodenal (i.d.) administration using the following
equation: Bioavailability = AUC (i.d.)/AUC (i.v.).
b Maximal concentration of zinc in serum.
*p < 0.05.
***p < 0.001.
ZINC COMPLEX OF L-CARNOSINE AND OTHER LIGANDS AS PROMISING
COMPOUNDS FOR CLINICAL USE
Features of zinc as an essential element. On one hand, the versatility in biological functions and low toxicity of zinc have been made clear by the results obtained in a wide range of study. Before discussing the possibility of the development of new drugs based on the complexes formed from zinc with L-carnosine and its related ligands, physiological features of zinc as a counterpart of the ligand and its complexes should be described briefly here. Functions of zinc in living systems may be summarized as follows: 1) formation, growth and metabolism of cells, healing of wounds; 2) activation and secretion of hormones; 3) maintenance of neurotransmission system and ability of memory; 4) maintenance of normal carbohydrate metabolism; 5) maintenance of normal lipid metabolism; 6) stabilization of cellular membranes; 7) maintenance of skin stability; 8) maintenance and activation of immune system; 9) maintenance of sensitivity in taste and smell; 10) maintenance of stability of retina and crystalline lens; 11) maintenance of normal alcohol metabolism; 12) reduction of hazardous effect of heavy metals. In these functions, zinc plays an essential role as zinc-containing enzymes in many cases or complexes formed with some components in biological systems. In conclusion, the significance of zinc in living systems is recognized to be the maintenance of balance and the adjustment of various physiological reactions in delicately controlled homeostatic systems, as in the human body. We can obtain a general view of recent progress on the study of zinc through some reviews [23, 24]. Zinc is regarded as an essential trace metal element that shows particular importance among other essential trace elements, such as Fe, Cu, Co, Mn, Mo, V, and Cr. These trace elements generally play their own specific role in living systems. On the contrary, the nature of the functions of zinc is different from those of other elements and related closely to wide varieties of physiological reactions.
Advantageous features of zinc in the above-mentioned functions, and hence favorable aspects in applications for medical use are well explained by the following physicochemical basic aspects of the character of zinc ion and its complexes. 1) Zinc ion has a small radius and acts as a Lewis acid and hence zinc ion can play an advantageous role as a catalyst in hydrolysis reactions. 2) The stability of zinc complexes with some ligands, those present in living system, is satisfactorily high but not too high, so that zinc is reactive in complexation. This means high probability of ligand-exchange reactions in zinc complexes in living systems. Irving-Williams stability order, that is proved applicable to various divalent metal complexes with common ligands, shows physicochemically that the stability of zinc complex is generally lower than that of copper and is comparable to that of nickel and much higher than that of calcium or magnesium. 3) The bivalent state is stable because of the fully occupied 3d-orbital, and it is maintained even in highly oxidizing and reducing environments. 4) Fairly high affinity is shown to the main coordination atoms, such as sulfur, nitrogen, and oxygen. This means high flexibility in the structure, coordination mode, and coordination number of the complexes produced.
These physicochemical features are particular to zinc and its complex. Consequently, these features shown by zinc and zinc complexes are firmly regarded as the bases of the versatility of zinc in biological systems. In addition, these features give rise to easy excretion, namely, prevention of the accumulation of zinc in a living system, and its low toxicity can be well explained by these features. In the human body, generally 2-3 g of zinc is present and about 15 mg per day is necessary for the maintenance of healthy condition.
Applicability of zinc complexes to clinical use. We have attempted to develop new drugs by the application of zinc and appropriate ligands, taking advantage of the above-mentioned favorable features of zinc and its complexes. Polaprezinc (L-CAZ) is our first success. In recent years, in connection with the hazardous effect of Helicobacter pyroli to gastrointestinal ulcer and its potent relation to cancer, combinations of some drugs aimed at its eradication from the stomach have been very actively studied. The fact that Polaprezinc (L-CAZ) shows an inhibitory effect against the growth of Helicobacter pylori deserves great attention [25]. The mechanism of this effect has not been clarified. Considering the effect of zinc on urease [26], which is excreted from Helicobacter pylori for its growth under the strongly acidic conditions in the stomach, the following inference is possible. It is well known that the active center of urease contains nickel ion, which is indispensable to the enzymatic activity. If nickel is replaced by zinc, urease is substantially inactivated. We can presume that the replacement of nickel by zinc occurs considering the comparable complex-forming ability of these two metal ions, and inactivation of urease may cause the inhibition of growth of Helicobacter pylori.
There have been few attempts to find applications of zinc complexes in medicine so far. Recently, zinc chelates synthesized from some sulfur-containing ligands were found to exhibit high affinity to lipid-rich regions and the possibility for the treatment of ischemic heart disease using these complexes was shown [27]. Special attention has been given recently to zinc gluconate lozenges for the relief of common cold symptoms. Further study is needed to clarify the role of zinc in this treatment [28, 29]. Roles of zinc in neurological, immunological, and endocrinological systems have been studied actively in recent years [30, 31]. The relation of zinc to Alzheimer's disease has been one of the interesting problems, although the effect of zinc compounds is still ambiguous [32]. In connection with aging, zinc is the most interesting trace metal element [33-36]. This means the complex of zinc with L-carnosine, which is effective in the control of aging, should be studied from various points of view. On one hand, remarkable low toxicity of zinc has been confirmed in clinical use of L-CAZ. Wide survey of examples of hazardous effects of zinc shows that the appearance of toxicity is limited to accidental ingestions of large amounts, and in most cases the toxicity was found to be reversible [23]. Judging from these reports and relevant findings in clinical use of rather large amounts of zinc, unfavorable side effects can be practically avoided by careful monitoring in general medical treatment.
Considering the advantageous features of L-carnosine and its complexes formed with zinc mentioned above, we will continue the study for the development of new drugs using these promising materials.
REFERENCES
1.Matsukura, T., Takahashi, T., Nishimura, Y.,
Ohtani, T., Sawada, M., and Shibata, K. (1990) Chem. Pharm.
Bull., 38, 3140-3146.
2.May, P. M., Linder, P. W., and Williams, D. R.
(1977) J. Chem. Soc. Dalton Trans., 588-595.
3.Berton, G., May P. M., and Williams, D. R. (1978)
J. Chem. Soc. Dalton Trans., 1488-1492.
4.Itoh, H., Yamane, T., Ashida, T., and Kakudo, M.
(1977) Acta Crystallogr., B33, 2959-2961.
5.Hara, H., Shibata, Y., Okuyama, T., Yamano, M.,
Okuyama, S., Kitamori, S., Okuyama, K., and Namiki, M. (1991) Ther.
Res., 12, 3239-3241.
6.Seiki, M., Aita, H., Mera, Y., Arai, K., Toyama,
S., Furuta, S., Morita, H., Hori, Y., Yoneta, T., and Tagashira, E.
(1992) Folia Pharmacol. Japon, 99, 255-263.
7.Boldyrev, A. A., Stvolinsky, S. L., Tyulina, O. V.,
Koshelev, V. B., Hori, N., and Carpenter, D. (1997) Cell. Molec.
Neurobiol., 17, 259-271.
8.Seiki, M., Ueki, S., Hori, Y., Aita, H., Morita,
H., Yoneta, T., and Tagashira, E. (1992) Ther. Res., 13,
885-891.
9.Lenz, G. R., and Martell, A. E. (1964)
Biochemistry, 3, 750-753.
10.Nishimura, Y., and Matsukura, T. (2000),
preparing manuscript to be submitted.
11.Seiki, M., Aita, H., Ueki, S., Yoneta, T.,
Takemasa, T., Hori, Y., Morita, H., Cyaki, K., and Tagashira, E. (1992)
Folia Pharmacol. Japon, 100, 165-172.
12.Arakawa, T., Satoh, H., Nakamura, A., Nakamura,
H., Ishikawa, M., and Kobayashi, K. (1988) Gastroenterology,
94, A12.
13.Arakawa, T., Satoh, H., Nakamura, A., Nebiki, H.,
Fukuda, T., Sakuma, H., Nakamura, H., Ishikawa, M., Seiki, M., and
Kobayashi, K. (1990) Digest. Dis. Sci., 35, 559-566.
14.Itoh, M., Tanaka, T., and Suzuki, Y. (1990)
Jap. J. Pharmacol., 52, 513-521.
15.Yoshikawa, T., Naito, Y., Tanigawa, T., Yoneta,
T., Oyamada, H., Ueda, S., Takemura, T., Sugino, S., and Kondo, M.
(1989) J. Clin. Biochem. Nutr., 7, 107-113.
16.Seiki, M., Ueki, S., Tanaka, Y., Soeda, M., Hori,
Y., Aita, H., Yoneta, T., Morita, H., Tagashira, E., and Okabe, S.
(1990) Folia Pharmacol. Japon, 95, 257-269.
17.Yoshikawa, T., Naito, Y., Tanigawa, T., Yoneta,
T., Yasuda, M., Ueda, S., Oyamada, H., and Kondo, M. (1991) Free
Rad. Res. Commun., 14, 289-296.
18.Morise, K. (1992) Jpn. Pharmacol. Ther.,
20, 235-244.
19.Hayakawa, A., and Inoue, M. (1992) Jpn.
Pharmacol. Ther., 20, 255-264.
20.Sano, H., Furuta, S., Toyama, Miwa, M., Ikeda,
Y., Suzuki, M., Sato, H., and Masuda, K. (1991) Arzneim.
Forsch., 41, 965-975.
21.Sandstroem, B. (1980) Naeringsforskning,
24, 110-118.
22.Evans, G. R., and Hahn, C. J. (1974) Adv. Exp.
Med. Biol., 48, 285-297.
23.Walsh, C. T., Sandstead, H. H., Prasad, A. S.,
Newberne, P. M., and Fraker, P. J. (1994) Environ. Health
Perspectives, 102, 5-46.
24.Favier, A. (1998) in Metal Ions in Biology and
Medicine (Collery, P., ed.) Vol. 5, John Libbey Eurotext,
Montrouge, pp. 164-168.
25.Raimsford, K. D., Goldie, J., and Hunt, R. H.
(1997) Pharm. Sci., 1997, 483-485.
26.Perez, G. H., Gower, C. B., and Blazer, M. J.
(1994) Infection and Immunity, 62, 299-302.
27.Saji, H., Kinoshita, T., Kubota, M., Kaneda, K.,
Akaike, A., Kikuchi, M., Kashii, S., Honda, Y., Iida, Y., Magata, Y.,
and Yokoyama, Y. (1997) S. T. P. Pharma Sci., 7,
92-97.
28.Mossad, S. B., Macknin, M. L., and Sharon, V.
(1996) Ann. Int. Med., 125, 81-88.
29.Macknin, M. L., Piedmonte, M., Calendine, C.,
Janosky, J., and Wald, E. (1998) J. Amer. Med. Associat.,
279, 1962-1967.
30.Ross, G. M., Shamovsky, I. L., Lawrance, G.,
Solc, M., Dostaler, S. M., Jimmo, S. L., Weaver, D. F., and Riopelle,
R. J. (1997) Nature Med., 3, 872-878.
31.Huang, E. D. (1997) Proc. Natl. Acad. Sci.
USA, 94, 13386-13387.
32.Multhaup, G., Bush, A. I., Dollwein, P., and
Masters, C. L. (1994) FEBS Lett., 355, 151-154.
33.Miyata, S., Okuno, T., Shimamura, Y., and Miyake,
T. (1987) Jpn. J. Geriat., 24, 272-277.
34.Fabris, N., and Mocchegiani, E. (1995) Aging
Clin. Exp. Res., 7, 77-93.
35.Tully, C. L., Snowdon, D. A., and Markesbery, R.
(1995) Neuroreport, 6, 2105-2108.
36.Fortes, C. (1995) Aging Clin. Exp. Res.,
7, 75-76.