REVIEW: Carnosine-Related Dipeptides in Neurons and Glia
S. De Marchis1, C. Modena1, P. Peretto1, A. Migheli2, F. L. Margolis3, and A. Fasolo1*
1Dipartimento di Biologia Animale e dell'Uomo, Universita' di Torino, Torino, Italy; fax: ++39-011-8124824; E-mail: fasolo@dba.unito.it2Dipartimento di Neuroscienze, Universita' di Torino, Torino, Italy
3Department of Anatomy and Neurobiology, University of Maryland, Baltimore, Maryland, USA
* To whom correspondence should be addressed.
Received October 28, 1999
Carnosine-related dipeptides have been demonstrated to occur in the nervous tissue of many vertebrates, including humans. Although several hypotheses have been formulated, to date their precise physiological role in the nervous system remains unknown. This article will review the studies on the presence and distribution of these dipeptides in the nervous system of different classes of vertebrates. It will focus on the most recent data on their cellular localization and potential functions in mammals. The studies on localization of carnosine-related dipeptides show a complex pattern of expression that involves both neuronal and glial cell types. The glial localization, widely distributed throughout the whole brain and spinal cord, includes a subset of both mature astrocytes and oligodendrocytes, whereas the neuronal localization is restricted to a particular type of neurons (the olfactory receptor neurons), and to restricted populations of putative migrating neurons and neuroblasts. There is no definitive demonstration of the function of these dipeptides in the various cell types. However, a wide array of evidence suggests that carnosine-related dipeptides could act as natural protective agents. Moreover, recent studies have suggested that, as previously postulated for the olfactory receptor neurons, in mature functional glial cells as well, carnosine-related dipeptides could be implicated in a neuromodulatory functional mechanism.
KEY WORDS: carnosine, carnosine-related compounds, mammalian nervous system, neurons, glia, olfactory bulb
Abbreviations: CNP) 2´,3´-cyclic nucleotide-3´-phosphodiesterase; CNS) central nervous system; GFAP) glial fibrillary acidic protein.
I. INTRODUCTION: OVERVIEW OF CARNOSINE-RELATED DIPEPTIDES IN
VERTEBRATE NERVOUS TISSUE
I.1. Biochemical Evidence
From the initial discovery of carnosine (beta-alanyl-L-histidine) [1] and the related dipeptides anserine (beta-alanyl-N1-methyl-L-histidine) [2, 3] and homocarnosine (gamma-aminobutyryl-L-histidine) [4], numerous biochemical studies have been carried out in many vertebrates, including humans, demonstrating their presence in the central nervous system (CNS) and in various sensory systems such as the olfactory and the visual systems.
I.2. Immunohistochemical Localization
In the last decade, the availability of specific antisera led to a more detailed investigation of the distribution and cellular localization of these dipeptides. Two rabbit polyclonal antisera raised against carnosine [5, 6] and one against anserine [6] have been produced and used in numerous immunohistochemical studies. The purified anti-anserine serum is monospecific and does not cross-react with the other related dipeptides; on the other hand, both the purified anti-carnosine sera cross-react with carnosine, homocarnosine, and anserine [5, 6]. The use of these sera raises the problem of discrimination among the different dipeptides, emphasizing that immunohistochemical observations always need to be interpreted cautiously and, where possible, should be supported by parallel chemical analyses.
I.3. Distribution in Different Vertebrate Classes
The occurrence and distribution of the different carnosine-related dipeptides is highly variable among different classes of vertebrates. Biochemical analyses carried out on the nervous system of lower vertebrates (osteichthyes, amphibia, and reptilia) demonstrated that primarily homocarnosine, and to a lesser extent carnosine, are present [7, 8]. Immunocytochemical studies showed that in the brain of reptilia and amphibia anura these dipeptides are localized in glial cells, whereas in urodele species the cellular localization is restricted to neuronal cells of several brain areas [8, 9]. In reptiles these dipeptides were also described to occur in the olfactory system associated with the olfactory receptor neurons, whereas in amphibia they were not detected in this cell population [8-10]. However, using a monoclonal antibody to mammalian carnosine synthetase [11], immunohistochemical reactivity was reported in a subpopulation of frog olfactory receptor neurons [12]. This occurred with only one of several independent monoclonals. Curiously, this is the only instance in which an immunohistochemical reaction for carnosine-synthase could be detected with this antibody (Margolis, unpublished observations).
In the retina of amphibians, where primarily carnosine has been detected [7], the immunoreactivity is localized in photoreceptors and bipolar cells. A few presumed amacrine and ganglion cells, as well as Muller cell endfeet were also described to be immunopositive [13, 14]. Other sensory receptors were found to contain carnosine-related dipeptides in different species: the hair cells of the semicircular canals of the inner ear of the frog [15] and the sensory cells of the lateral line organ of Xenopus [16]. In addition, significant levels of these dipeptides were detected in the trout saccular nerve [17].
In the nervous system of birds, both anserine and carnosine are present, the former associated with glial cells in the brain and the latter restricted to the olfactory receptor neurons [6]. Furthermore, very high levels of anserine have been reported to occur in the avian retina [7]. Consistent with these chemical studies, immunocytochemical analysis of the chick retina demonstrates that anserine is largely restricted to the Muller cells (Sharma and Margolis, unpublished observations). Anserine is absent in the mammalian nervous system, which only contains homocarnosine and carnosine [6, 7, 18, 19]. A number of analytical studies performed on different mammalian species established that carnosine is highly concentrated in the olfactory bulb, whereas lower concentrations of carnosine and homocarnosine occur in the brain and in the spinal cord [4, 20-23]. Low levels of homocarnosine are also present in the mammalian retina [7]. The cellular localization of the carnosine-related dipeptides in the nervous system of mammals involves both neurons and glial cells [6]. The most recent studies on the presence and function of these dipeptides in the mammalian nervous system show a more detailed picture of their distribution in vivo and provide new evidence of their localization, synthesis, uptake, and potential role in vitro and in vivo. In the following sections we will describe in detail the distribution of carnosine-related dipeptides in different cellular compartments of the mammalian nervous system, together with the potential roles suggested for these dipeptides.
II. CARNOSINE-RELATED DIPEPTIDES IN THE MAMMALIAN NERVOUS
SYSTEM
Multiple approaches in the study of the distribution and cellular localization of carnosine and homocarnosine in the nervous system of mammals have shown the existence of a complex pattern of distribution involving different cell types. The synthesis of the two dipeptides is carried out by a single enzymatic activity, carnosine synthetase [24-26], which has been demonstrated to be present in both neurons [27-29] and glia [30-32]. However, between these two cellular types big differences were observed concerning the amount and the regional distribution of those cells containing carnosine and/or homocarnosine and on the relative abundance of these dipeptides.
II.1. Carnosine-Related Dipeptides in Neurons: Distribution and Cellular Localization
Studies carried out in the mature mammalian nervous system have demonstrated that the localization of carnosine-related dipeptides in neurons primarily involves a population of cells located outside the brain: the olfactory receptor neurons. Further investigations show that these dipeptides are also present within the brain in populations of putative neurons and neuroblasts that share the features of migratory cells. Actually, during fetal and postnatal development, the presence of these dipeptides was observed in cells migrating from the nasal region into the brain. Moreover, their occurrence has been recently demonstrated in migrating neuroblasts of the sub-ependymal layer (SEL).
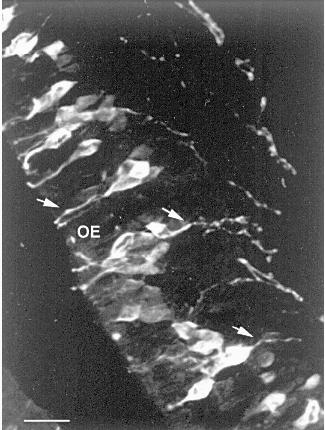
II.1.1. The olfactory receptor neurons. Since 1974, when chromatographic and electrophoretic techniques demonstrated that carnosine is present in the olfactory bulb and nasal olfactory epithelium of rodents at concentrations exceeding that previously reported for any brain region [21, 33], most of the studies performed on the carnosine-related dipeptides in the mammalian nervous system have been focused on their occurrence and possible function in the olfactory system. The olfactory epithelium is the site of the cell bodies of the olfactory receptor neurons (ORNs), which project their axons to the glomerular layer of olfactory bulb where they synapse with the dendrites of mitral cells and other second-order neurons. Studies carried out after olfactory bulbectomy or peripheral deafferentation indicated that carnosine is localized within the ORNs [21, 34, 35]. These results were subsequently confirmed by other biochemical [29], autoradiographic [29] and immunohistochemical studies showing that the mature ORNs contain carnosine in their perikarya and cell processes (Fig. 1), including the axonal projections to the main and accessory olfactory bulb [5, 6, 36]. The occurrence of carnosine in ORNs recovers after bulbectomy [37] and is present in cultured olfactory receptor cells [38] and in explants of olfactory neuroepithelium [39], indicating that olfactory neurons can express this dipeptide in the absence of specific connections with their target. In contrast with these numerous experimental demonstrations, a recent in vitro study [40] has suggested that carnosine is synthesized by the ensheathing cells of the olfactory bulb and not by the olfactory neurons.
Studies on carnosine metabolism have shown that a very high synthase activity is present in the olfactory bulb and epithelium [27]. The response of the carnosine synthetase activity to denervation [27, 35] suggested that this enzyme is localized in the ORNs. Indeed, following irrigation of the mucosa with radiolabeled beta-alanine and histidine, the precursors are rapidly taken up and converted to carnosine, which is transported to the olfactory bulb by axonal flow [41]. A wide array of biochemical and neurochemical data, including the detection of high levels of carnosine, carnosine synthetase, and the degradative enzyme, carnosinase in the olfactory system of several mammalian species [21, 27, 29, 33, 35] and the specific localization of carnosine in the ORNs [5, 6] and in receptor cells of other sensory systems [13-15], together with the selective binding of carnosine to membrane preparations from the olfactory bulb [42, 43], led to the hypothesis that the dipeptide could participate in neurotransmission. In spite of these experimental results, electrophysiological data are equivocal, either supporting [44-48] or not [49-52] a role for carnosine as the neurotransmitter of the ORNs. For example, application of carnosine to the olfactory bulb glomerular layer of rabbits has been shown to increase the frequency of evoked potentials on the stimulation of the lateral olfactory tract and to produce a sustained oscillation in electroencephalographic activity [44]. It has also been demonstrated that potassium-induced depolarization releases carnosine from olfactory bulb synaptosomes in a calcium-dependent manner [46], and recent studies demonstrated that the application of carnosine to organotypic slice cultures of the olfactory bulb induce inward current responses [48]. On the other hand, no effect on mitral cells has been reported in rat olfactory bulb injected with carnosine by microiontophoresis [49], and similar results were obtained by Nicoll et al. [51] using in vitro preparations of turtle and frog olfactory bulb, although amphibia lack endogenous olfactory carnosine [8-10].Fig. 1. Carnosine-like immunoreactive olfactory receptor neurons. Arrows indicate an immunopositive apical dendrite and two axonal processes. OE, olfactory epithelium. Scale bar: 20 µm.
Previous biochemical studies have shown that carnosine is localized in the cytosol of mouse olfactory bulb and epithelium, not associated with synaptic vesicles [50]. However, a more recent ultrastructural study, performed using a post-embedding immunogold technique, has demonstrated that carnosine is specifically co-localized with glutamate and zinc and/or copper in the synaptic terminals of olfactory receptor neurons [47]. These results strongly suggest that this dipeptide, which can chelate divalent metals [53], may interact with glutamate or zinc/copper in the transmission of olfactory stimuli. This view was recently supported by an in vitro study performed on cultured olfactory bulb neurons [54]. The authors did not observe any direct or glutamate-evoked effects of carnosine on membrane properties of olfactory bulb neurons, but they showed how potently carnosine inhibits the effects of copper, and to a lesser extent zinc, on amino acid receptors and synaptic transmission. These data suggest that this dipeptide may act indirectly as a neuromodulator by influencing the effects of zinc and copper on neuronal excitability.
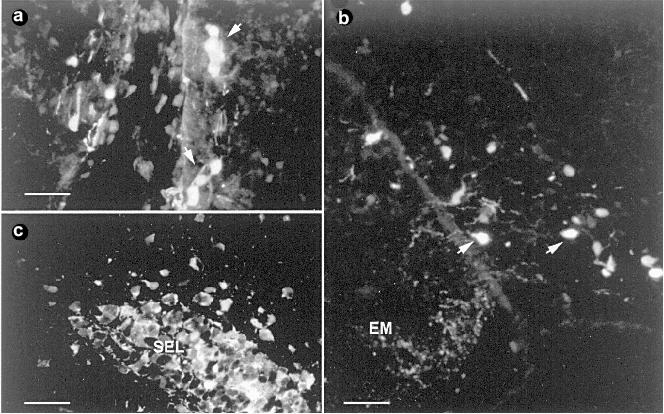
II.1.2. Migrating cells deriving from the olfactory placode. From an ontogenic point of view, the occurrence of carnosine in the rodent nervous system was biochemically detected starting from embryonic day 16 in rat olfactory tissue [39]. At approximately the same time (from fetal day 14 to day 19), clusters or single cells immunopositive for anti-carnosine were described in mice on the outer medial and ventral aspect of the olfactory bulbs, along the olfactory fiber tracks [55]. These cells located outside the olfactory epithelium were identified as migratory neurons. Actually, during early development the mammalian olfactory system is characterized by the migration of several populations of neurons from the olfactory placode to the brain (reviewed in [56]). Such cells migrate in heterogeneous groups following a defined pathway from their origin to the olfactory bulb and eventually to the hypothalamic and septal areas. Among these cells, there are neurons expressing the decapeptide gonadotropin-releasing hormone (GnRH), which are committed to form the gonadotropic compartment of the hypothalamus. Carnosine-like immunopositive cells outside the olfactory epithelium were also reported in neonatal (P1-P7) pups of the marsupial Monodelphis domestica (Fig. 2a) [57]. Although the fate and the final phenotype of such cells is not clear, it has been suggested that these transitory cells could play a role in guiding the olfactory fibers and in the establishment of their final connections in the olfactory bulb. However, recent findings have brought new evidence demonstrating the occurrence of carnosine-expressing neuronal-like cells and fibers in different brain regions of postnatal rats [58].
The presence of such peptide immunoreactivity was observed in fibers running throughout the ventro-medial aspect of the olfactory bulb, along the olfactory peduncle, and in more caudal regions of the basal prosencephalon, with a high concentration of terminals in the median eminence. Developmental analysis showed that this staining is transitory; in fact, after postnatal day 21 only rare carnosine-like immunoreactive processes are found in these same regions. In addition, during the same period of time (from postnatal day 1 to postnatal day 21) a strong carnosine-like immunoreactivity was observed in isolated or small groups of putative neuronal cells in the ventro-medial hypothalamus (Fig. 2b) and in the subfornical organ. These cells were considered to derive from the olfactory placode, and the hypothesis was formulated that they could contribute not only to drive the organization of the prosencephalon, but also to the formation of the central neuroendocrine system.Fig. 2. Carnosine-like immunoreactive cells. a) Clusters of immunoreactive cells (arrows) in the medial rostral telencephalon of a P1 opossum Monodelphis domestica, coronal section. Scale bar: 45 µm. b) Coronal section of P3 rat brain at the level of the tuberal hypothalamus. Arrows indicate two immunopositive cell bodies. EM, median eminence. Scale bar: 45 µm. c) Coronal section of the adult rat forebrain in the horizontal arm of the SEL (sub-ependymal layer) rostral extension. Scale bar: 30 µm.
II.1.3. The newly generated cells of the sub-ependymal layer (SEL). Recent studies reported the occurrence of carnosine-related dipeptides in the SEL of adult rodents. The SEL is a remnant of the embryonic subventricular germinal zone that in the adult persists within a restricted area of the forebrain corresponding to the anterior part of the lateral ventricles and to the primitive olfactory ventricles. The persistence of this germinal zone in the brain of adult rodents has been known for many years [59, 60]. Recently, interest in this region has increased as there are indications that it is a putative stem cell compartment of the mature central nervous system [61, 62]. The SEL involves two distinct components: astrocytic cells forming glial tubes and chains of newly generated cells that migrate tangentially within the glial tubes from the lateral ventricles towards the more internal layer of the olfactory bulb. Upon arrival in the olfactory bulb, they continue their migratory pathway outside the tubes in an isolated radially oriented manner to reach more external layers where they are thought to differentiate into neurons (reviewed in [63]).
Recent immunohistochemical studies performed on the brain of rats and mice have demonstrated the occurrence of carnosine-related dipeptides in both components of the SEL starting from the third postnatal week (Fig. 2c) [64]. In particular, double labeling with markers that specifically identify the migrating neuroblasts of the SEL (PSA-NCAM, stathmin, class III beta-tubulin) demonstrated that carnosine is co-expressed in these cells. Moreover, the colocalization of carnosine-like immunoreactivity with 5-bromo-2´-deoxyuridine (BrdU), immunocytochemically detected after systemic administration, confirmed that these are newly generated cells. Interestingly, unlike the other markers of the migrating neuroblasts of the SEL, the occurrence of carnosine-like immunoreactivity is limited to the tangentially oriented part of the migratory route, and no reaction was observed in the isolated elements migrating radially outside the glial tubes. This transient expression was thought to be related to the particular type of tangential chain migration occurring in the SEL [64].
II.2. Carnosine-Related Dipeptides in Central Glia
Apart from the olfactory system and the migrating elements reported above, where the occurrence of carnosine-related dipeptides was described in cells belonging to the neuronal lineage, in the rest of the CNS the occurrence of these dipeptides is confined to glial cells. Unlike the restricted neuronal localization, their presence in glial cells is widely distributed within the brain and in the spinal cord.
II.2.1. In vitro models: biosynthesis, uptake, and release. It is well documented that unlike the olfactory system, where high levels of carnosine have been detected, the CNS of mammals contains much lower concentrations of carnosine and homocarnosine [21, 33, 34, 65]. One of the first attempts to obtain information on the metabolic compartmentalization of the dipeptides in the CNS was performed by studying their metabolism in neuronal and glial cell-enriched fractions of rabbit brain [28]. The results obtained suggested that carnosine and homocarnosine synthetic activities are preferentially located in neurons.
However, in contrast to these findings, the use of in vitro models showed that glial cells might biosynthesize these dipeptides. Carnosine and homocarnosine were first demonstrated to be synthesized by the rat glioma cell line C6 [30], and subsequently by astroglia-rich primary cultures of newborn mouse brain [31]. When incubated with radiolabeled precursor, these cells were able to synthesize carnosine. By contrast, only low levels of homocarnosine were reported to be produced, probably due to a difference in the affinities of the two precursors (beta-alanine and gamma-aminobutyric acid) for carnosine synthetase, as previously demonstrated for the synthase from olfactory bulb [26]. The various dipeptides, once synthesized, were released at different rates in the culture medium. Further studies indicated that the in vitro synthesis of carnosine is regulated by the composition of the culture medium, and the presence of a high concentration of dibutyryl cyclic AMP was demonstrated to induce a depression in the synthesis of the dipeptide [66].
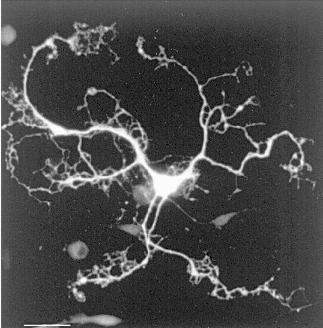
Since primary cultures of glial cells consist of a mixed population of cells, this model, even if of fundamental importance in the demonstration of the potential of the glial cells to synthesize carnosine and homocarnosine, did not allow discrimination between the possible different cell types responsible for the processes of synthesis, release, and uptake. In the last few years, the generation of selective enriched cultures of oligodendrocytes and astrocytes has led to the demonstration that carnosine synthesis is restricted to oligodendrocytes, and carnosine uptake, but not synthesis, to astrocytes [32]. Interestingly, these results were confirmed by others who showed that carnosine immunolocalization obtained in vitro was restricted to oligodendrocytes, further indicating that this dipeptide is a natural component of these cells (Fig. 3), whereas no positivity was obtained in cultures enriched in astrocytes [67]. Moreover, in the enriched culture that contained oligodendrocytes at different maturation stages, only a subpopulation of cells was immunopositive. The same cells were immunostained with markers specific for fully differentiated oligodendrocytes such as myelin basic protein (MBP) and 2´,3´-cyclic nucleotide 3´-phosphodiesterase (CNP), but not with markers specific for oligodendrocyte precursors (A2B5 and LB1), thus suggesting that only mature oligodendrocytes contain carnosine-related peptides.
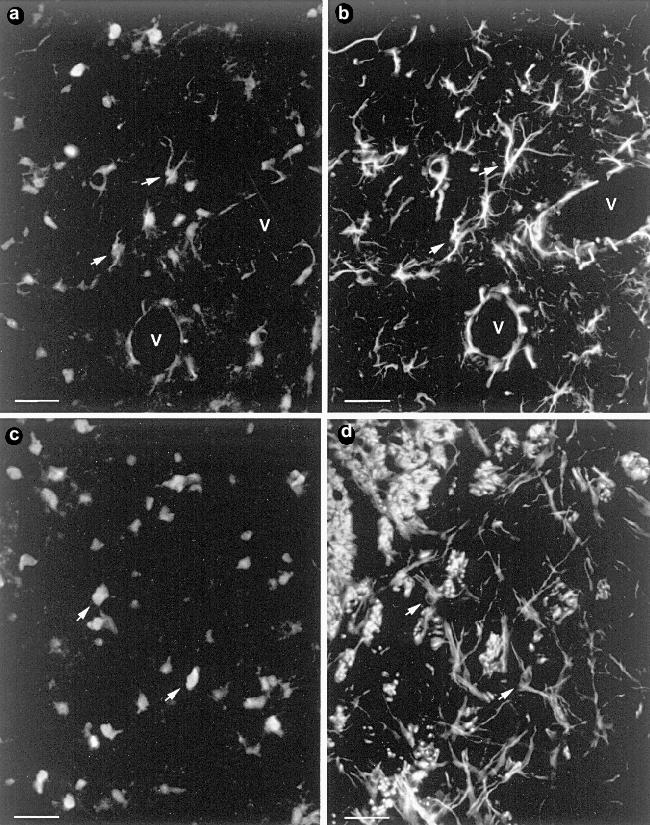
II.2.2. In vivo distribution and cellular localization. The first demonstration of the glial localization of carnosine-related dipeptides in the mammalian nervous system was performed by Biffo et al. [6] with an extensive mapping of the carnosine-like immunoreactivity in the mouse brain. Cells exhibiting carnosine-like immunoreactivity were described to be widely distributed throughout the mouse brain. These cells were identified as glial cells based on their morphological appearance and anatomical distribution. In recent studies, carried out in vivo on the brain of adult rats [67], the glial nature of the carnosine-like immunoreactive cells has been confirmed by means of simultaneous double staining methods using anti-carnosine serum in combination with different glial markers specific for mature astrocytes and oligodendrocytes.Fig. 3. Carnosine-like immunoreactive oligodendrocyte in culture. Scale bar: 42 µm.
A conspicuous number, but not all the glial cells localized in the white and gray matter of the brain, were detected to be immunopositive when treated with anti-carnosine serum. Process-bearing carnosine-like immunoreactive cells were double labeled for GFAP (glial fibrillary acidic protein), whereas other cells in which the staining for carnosine was restricted to the round cellular bodies with apparently unstained processes, were GFAP-negative, but they were stained by antibodies specific for the oligodendrocyte marker RIP (Fig. 4). These results demonstrated that in contrast to the in vitro localization [67], in the brain the carnosine-related dipeptides are not restricted to a unique cellular type but are present in subpopulations of both mature astrocytes and oligodendrocytes. Further studies showed that the spinal cord displays a similar pattern of staining [58], whereas no immunoreactivity was observed in the glia of the peripheral nervous system [67].Fig. 4. Coronal section of adult rat forebrain. Double simultaneous immunostaining with anti-carnosine (a, c) and anti-GFAP (b) or RIP antibodies (d). Some of the double-labeled cells are indicated with arrows. V, blood vessels. Scale bar: 28 µm.
In the brain, among the GFAP-immunopositive cells, the occurrence of the dipeptides has been detected in certain cell populations which are remnants of the radial glia, such as the cerebellar Bergmann glia [6], or display a radial-like morphology such as certain glial cells in the hypothalamic supraoptic nuclei [68]. Other cell populations that show cellular elements highly positive for carnosine [68] are those formed by ependymal cells, which have been recently suggested to play a potential role as a stem cell compartment in the adult CNS [69], and tanycytes, a particular type of ependymal cells which line certain parts of the ventricular cavities [70]. Moreover, carnosine-like immunoreactivity is highly expressed in the astrocytes forming the glial tubes of the SEL [64] (see also section II.1.3).
A developmental analysis performed on the CNS of rats on the distribution of carnosine-related dipeptides revealed that the glial immunostaining for these substances evolves during the first three postnatal weeks of life, spreading along a caudo-rostral gradient from the spinal cord to the forebrain (Fig. 5) [58]. It is well known that during this same period the differentiation of both types of macroglial cells into mature functional elements take place [71]. Thus, the developmental spatio-temporal pattern observed for carnosine-like immunoreactivity strongly suggests a correlation between the wave of glial maturation and the expression of carnosine-related dipeptides. Interestingly, it has been reported that the carnosine-like immunoreactivity appears sequentially in the two principal types of glial cells and is first evident in subpopulations of RIP-immunopositive mature oligodendrocytes [58]. This observation strongly supports the hypothesis that as reported in vitro, also in vivo oligodendrocytes are able to synthesize carnosine and/or homocarnosine.
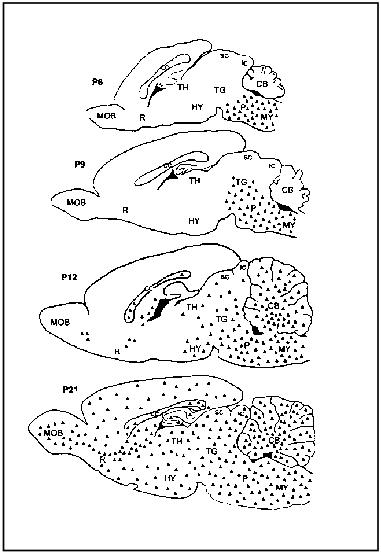
Preliminary data on the immunohistochemical localization of carnosine-related dipeptides in the human brain reveal the presence of such substances in glial-like cells of the subcortical white matter (Fig. 6). Based on their morphological appearance these immunopositive cells might be ascribed to the oligodendrocyte type. However, in contrast to our findings, a previous report describing the localization of homocarnosine in humans pointed out that its occurrence is restricted to neuronal cell bodies and fibers [72]. Immunohistochemical results on human tissues are often difficult to interpret because of poor tissue preservation; therefore, false positive reactions are possible. Further investigations, using immunogen adsorbed anti-carnosine as a control and analyzing different regions of the human brain, could contribute to a better understanding of the real phenotype of the carnosine-like immunopositive cells.Fig. 5. Schematic drawings illustrating the gradual development of carnosine-like immunoreactivity (triangles) in glial cells in the rat brain during the first three postnatal weeks. CB, cerebellum; cc, corpus callosum; Cx, cortex; HY, hypothalamus; ic, inferior colliculus; MOB, main olfactory bulb; MY, medulla; P, pons; R, rinencephalon; sc, superior colliculus; TG, tegmentum; TH, thalamus.
II.2.3. Possible functions. Several studies performed on the functions of carnosine-related dipeptides have demonstrated that these substances are able to act in different but correlative ways as protective agents (as antioxidant, metal chelator, free radical scavenger, and inhibitor of protein glycosylation). Carnosine has been reported to have a beneficial effect on the growth, morphology, and longevity of cultured human fibroblasts of the MRC-5 and HFF-1 lines [73]. In addition, L-carnosine, but neither D-carnosine nor a variety of histidine containing dipeptides, has a reversible, anti-mitotic effect on cultured L-6 cells (Morgan and Margolis, unpublished observations). Moreover, it has been recently shown that carnosine protects rat cerebellar granular cells from free radical damage in vitro [74]. A protective action of carnosine-related dipeptides was also demonstrated on the toxic effects of a truncated form of the neurotoxic beta-amyloid peptide, betaA 25-35 (that is implicated in cerebral vascular dysfunction and Alzheimer's disease) on rat brain vascular endothelial cells in culture [75].Fig. 6. Section of human brain immunostained with anti-carnosine and counterstained with toluidine blue. Carnosine-like immunoreactive cells (arrows) are present in the subcortical white matter. Scale bar: 32 µm.
These properties are of particular interest considering the localization of carnosine-related dipeptides in mature glial elements of the central nervous system. Glial cells play a variety of roles in the nervous system providing structural and trophic support to neurons, and participating with endothelial cells in the formation of the blood-brain barrier. Thus, it is likely that these cells are able to produce and release substances such as the carnosine-related dipeptides with the potential role of natural protective agents to prevent several types of brain damage. However, the recent studies demonstrating a Ca2+-dependent glutamate-receptor mediated release of carnosine by cultured oligodendrocytes [76] make it possible to postulate an additional role for this substance in the extracellular glia-neuron cell interaction. Oligodendrocytes as well as astrocytes express several neurotransmitter receptors and respond to glutamate with depolarization and an increase in intracellular Ca2+ [77-79]. In addition to these effects, recent observations demonstrated that stimuli producing an elevation in Ca2+ levels in astrocytes lead to the release of glutamate, which in turn regulates neuronal Ca2+ levels and synaptic neurotransmission [80]. These findings, in addition to the well-known roles played by glial cells in neurotransmission by supplying neurotransmitter precursors and inactivating neurotransmitters once they have been released (reviewed in Araque et al., [81]), suggest that astrocytes may actively participate in the information processing. Thus, as previously suggested for olfactory receptor neurons (see section II.1.1.), the occurrence of carnosine-related dipeptides in mature functional glial cells could be implicated in a neuromodulatory functional mechanism.
III. CONCLUDING REMARKS
In the last decade, several studies have been performed to understand the biological functions played by carnosine-related dipeptides in the nervous system. Although many theories have been proposed about a wide range of properties that enable them to act as antioxidants, metal chelators, free radical scavengers, inhibitors of protein glycosylation, and neuromodulators, their precise physiological role remains unknown. A correlation between the information obtained from functional studies and the analysis of the cell types expressing carnosine-related dipeptides may help us to achieve a better understanding of the biological roles played by these molecules. In this context, we have reviewed the most important studies on their distribution and cellular localization in mammalian nervous tissues, showing the occurrence of a complex pattern of distribution involving both neuronal and glial cell types. Interestingly, in spite of the heterogeneity among the different cellular types expressing carnosine-related dipeptides, the analysis of their characteristics demonstrated that interesting similarities occur between some of them.
The neuronal localization is mainly represented by the expression of high levels of carnosine in the mature primary olfactory receptor neurons, a particular type of neuronal cells characterized by their ability to be replaced from precursor cells throughout the animal's life [82]. In this system, several studies suggested a role for carnosine as a neuromodulator of olfactory function. Although definitive evidence supporting such an activity has not been provided to prove this role, recent studies on glial cells demonstrated a glutamate-receptor mediated release of this substance, supporting its role in mechanisms of extracellular glia-neuron cell interaction. Apart from the potential role in neuromodulatory functional mechanisms, the occurrence of carnosine in the olfactory receptor neurons, which are directly subjected to environmental insults, could be related to a possible neuroprotective function.
The presence of carnosine-related dipeptides has been recently demonstrated in the SEL of adult rodents, another area of the nervous system wherein neurogenesis continues during adulthood. Interestingly, their occurrence is also reported in ependymal cells, which have been supposed to represent, together with the SEL precursors, the potential stem cell compartment of the adult brain. Although intriguing, the occurrence of a direct link between the presence of these dipeptides and processes of neuronal plasticity has not been definitively demonstrated. Furthermore, the pattern of expression of carnosine-related dipeptides in the neuroblasts of the SEL led to the hypothesis that, rather than being involved in processes of cell renewal, their occurrence could be related to the particular type of tangential migration occurring therein. Interestingly, carnosine-related dipeptides have also been detected in another small population of putative migrating neurons present in a restricted area of the prosencephalon during fetal and postnatal development.
Unlike the restricted neuronal localization, the expression of carnosine-related dipeptides in glial and ependymal cells is widely distributed throughout the brain and spinal cord. Studies performed in adults and during postnatal development demonstrated that the expression of these dipeptides occurs in a subset of fully differentiated glial cells, involving both oligodendrocytes and astrocytes. The multiple approaches used to address the study of the biological functions of these dipeptides showed that they posses a wide range of properties enabling them to prevent several types of brain damage. This led to the hypothesis that their localization in glial cells, which are critically involved in functional activities of the brain, may correlate with the level of their metabolic activity and of the related oxidative metabolism.
We wish to thank Glauco Tarozzo for providing us with an image of carnosine-immunoreactive migrating cells in the opossum Monodelphis domestica. We also thank Claudio Gendusa for valuable technical assistance. This work was supported by grants from the University of Torino, CNR, and Compagnia di San Paolo (A.F.), by the University of Torino fellowship (C.M.), and by NIH grants DC 03112 and DC 03904 (F.L.M.).
REFERENCES
1.Gulewitsch, W., and Amiradzibi, S. (1900) Ber.
Dtsch. Chem. Ges., 33, 1902-1903.
2.Ackermann, D., Timpe, O., and Poller, K. (1929)
Z. Physiol. Chem., 183, 1.
3.Tolkatschevskaya, N. (1929) Hoppe-Seyler Z.
Physiol. Chem., 185, 538.
4.Pisano, J. J., Wilson, J. D., Cohen, L., Abraham,
D., and Udenfriend, J. (1961) J. Biol. Chem., 236,
499-502.
5.Sakai, M., Yoshida, M., Karasawa, N., Teramura, M.,
Ueda, H., and Nagatsu, I. (1987) Experientia, 43,
298-300.
6.Biffo, S., Grillo, M., and Margolis, F. L. (1990)
Neurosci., 35, 637-651.
7.Margolis, F. L., and Grillo, M. (1984)
Neurochem. Int., 6, 207-209.
8.Artero, C., Marti', E., Biffo, S., Mulatero, B.,
Andreone, C., Margolis, F. L., and Fasolo, A. (1991) Neurosci.
Lett., 130, 182-186.
9.Artero, C., Mulatero, B., Biffo, S., Andreone, C.,
Gozzo, S., Margolis, F. L., and Fasolo, A. (1991) Brain Behav.
Evol., 37, 168-178.
10.Margolis, F. L. (1980) The Role of Peptides in
Neuronal Function (Baker, J. L., and Smith, T., eds.) Dekker, N.
Y., pp. 545-572.
11.Margolis, F. L., Grillo, M., Hempstead, J., and
Morgan, J. I. (1987) J. Neurochem., 48, 593-600.
12.Crowe, M. J., and Pixley, S. K. (1991) Brain
Res., 538, 147-151.
13.Sassoe'-Pognetto, M., Cantino, D., and Fasolo, A.
(1992) Brain Res., 578, 261-268.
14.Panzanelli, P., Cantino, D., and
Sassoe'-Pognetto, M. (1997) Brain Res., 758, 143-152.
15.Panzanelli, P., Valli, P., Cantino, D., and
Fasolo, A. (1994) Brain Res., 662, 293-296.
16.Mroz, E. A., and Sewell, W. F. (1989) Hear.
Res., 38, 141-162.
17.Drescher, M. J., and Drescher, D. G. (1991) J.
Neurochem., 56, 658-664.
18.Nadi, N. S., and Margolis, F. L. (1978)
Analyt. Biochem., 91, 180-185.
19.Wideman, J., Brink, L., and Stein, S. (1978)
Analyt. Biochem., 86, 670-678.
20.Abraham, D., Pisano, J. J., and Udenfriend, S.
(1962) Arch. Biochem. Biophys., 99, 210-213.
21.Margolis, F. L. (1974) Science,
184, 909-911.
22.Osborne, N. N., Wu, P. H., and Neuhoff, V. (1974)
Brain Res., 74, 175-181.
23.Cairns, M. T., Miller, D. J., and O'Dowd, J. J.
(1988) J. Physiol., 407, 51.
24.Kalyankar, G., and Meister, A. (1959) J. Biol.
Chem., 234, 3210-3218.
25.Skaper, S. D., Das, S., and Marshall, F. D.
(1973) J. Neurochem., 21, 1429-1445.
26.Horinishi, H., Grillo, M., and Margolis, F. L.
(1978) J. Neurochem., 31, 909-919.
27.Harding, J. W., and Margolis, F. L. (1976)
Brain Res., 110, 351-360.
28.Ng, R. H., Marshall, F. D., Henn, F. A., and
Sellstrom, A. (1977) J. Neurochem., 28, 449-452.
29.Burd, G. D., Davis, B. J., Macrides, F., Grillo,
M., and Margolis, F. L. (1982) J. Neurosci., 2,
244-255.
30.Bauer, K., Salnikow, J., DeVitry, F.,
Tixier-Vidal, A., and Kleinkauf, H. (1979) J. Biol. Chem.,
254, 6402-6407.
31.Bauer, K., Hallermayer, K., Salnikow, J.,
Kleinkauf, H., and Hamprecht, B. (1982) J. Biol. Chem.,
257, 3593-3597.
32.Hoffmann, A. M., Bakardjiev, A., and Bauer, K.
(1996) Neurosci. Lett., 215, 29-32.
33.Neidle, A., and Kandera, J. (1974) Brain
Res., 80, 359-364.
34.Ferriero, D., and Margolis, F. L. (1975) Brain
Res., 94, 75-86.
35.Harding, J. W., Graziadei, P. P. C., Monti
Graziadei, G. A., and Margolis, F. L. (1977) Brain Res.,
132, 11-28.
36.Sakai, M., Ashihara, M., Nishimura, T., and
Nagatsu, I. (1990) Acta Otolaryngol. (Stockh.),
109, 450-453.
37.Biffo, S., Sassoe'-Pognetto, M., di Cantogno, V.
L., Perroteau, I., and Fasolo, A. (1992) Eur. J. Neurosci.,
4, 1398-1406.
38.Perroteau, I., Biffo, S., Tolosano, E., Tarozzo,
G., Bovolin, P., Vaudry, H., and Fasolo, A. (1994) NeuroReport,
5, 569-572.
39.Margolis, F. L., Grillo, M., Kawano, T., and
Farbman, A. I. (1985) J. Neurochem., 44, 1459-1464.
40.Bakardjiev, A. (1997) Neurosci. Lett.,
227, 115-118.
41.Margolis, F. L., and Grillo, M. (1977)
Neurochem. Res., 2, 507-519.
42.Hirsch, J. D., Grillo, M., and Margolis, F. L.
(1978) Brain Res., 158, 407-422.
43.Hirsch, J. D., and Margolis, F. L. (1979)
Brain Res., 174, 81-94.
44.Gonzales-Estrada, M. T., and Freeman, W. J.
(1980) Brain Res., 202, 373-386.
45.Tonosaki, K., and Shibuya, T. (1979) Brain
Res., 167, 180-184.
46.Rochel, S., and Margolis, F. L. (1982) J.
Neurochem., 38, 1505-1514.
47.Sassoe'-Pognetto, M., Cantino, D., Panzanelli,
P., Verdun di Cantogno, L., Giustetto, M., Margolis, F. L., de Biasi,
S., and Fasolo, A. (1993) NeuroReport, 5, 7-10.
48.Kanaki, K., Kawashima, S., Kashiwayanagi, M., and
Kurihara, K. (1997) Neurosci. Lett., 231, 167-170.
49.MacLeod, N. K., and Straughan, D. W. (1979)
Exp. Brain Res., 34, 183-188.
50.Harding, J. W., and O'Fallon, J. V. (1979)
Brain Res., 173, 99-109.
51.Nicoll, R. A., Alger, B. E., and Jahr, C. E.
(1980) Proc. Roy. Soc. Lond., (B) 210, 133-149.
52.Frosh, M. P., and Dichter, M. A. (1984) Brain
Res., 290, 321-332.
53.Quinn, P. J., Boldyrev, A. A., and Formazuyk, V.
E. (1992) Mol. Aspects Med., 13, 379-444.
54.Trombley, P. Q., Horning, M. S., and Blakemore,
L. J. (1998) NeuroReport, 9, 3503-3507.
55.Tarozzo, G., Peretto, P., Perroteau, I.,
Andreone, C., Varga, Z., Nicholls, J. G., and Fasolo, A. (1994) Ann.
Endocrinol., 55, 249-254.
56.Tarozzo, G., Peretto, P., and Fasolo, A. (1995)
Zool. Sci., 72, 367-383.
57.Tarozzo, G., Peretto, P., Biffo, S., Varga, Z.,
Nicholls, J. G., and Fasolo, A. (1995) Proc. Roy. Soc. Lond.,
(B) 262, 95-101.
58.De Marchis, S., Modena, C., Giffard, C., Peretto,
P., and Fasolo, A. (1998) Soc. Neurosci. Abstr., 24, No.
52-28.12.
59.Smart, I. (1961) J. Comp. Neurol.,
116, 325-338.
60.Altman, J. (1969) J. Comp. Neurol.,
137, 433-458.
61.Morshead, C. M., Reynolds, B. A., Craig, C. G.,
McBurney, M. W., Staines, W. A., Morassutti, D., Weiss, S., and van der
Kooy, D. (1994) Neuron, 13, 1071-1082.
62.Reynolds, B. A., and Weiss, S. (1996) Dev.
Biol., 175, 1-13.
63.Peretto, P., Merighi, A., Fasolo, A., and
Bonfanti, L. (1999) Brain Res. Bull., 49, 221-243.
64.Peretto, P., Bonfanti, L., Merighi, A., and
Fasolo, A. (1998) Neurosci., 82, 527-542.
65.O'Dowd, J. J., Cairns, M., Robins, D., and
Miller, D. J. (1990) J. Neurochem., 55, 446-452.
66.Schulz, M., Hamprecht, B., Kleinkauf, H., and
Bauer, K. (1989) J. Neurochem., 52, 229-234.
67.De Marchis, S., Melcangi, R. C., Modena, C.,
Cavaretta, I., Peretto, P., Agresti, C., and Fasolo, A. (1997)
Neurosci. Lett., 237, 37-40.
68.Bonfanti, L., Peretto, P., de Marchis, S., and
Fasolo, A. (1999) Prog. Neurobiol., 56, 1-22.
69.Johansson, C. B., Momma, S., Clarke, D. L.,
Risling, M., Lendahl, U., and Frisen, J. (1999) Cell, 96,
25-34.
70.Flament-Durand, J., and Brion, J. P. (1985)
Int. Rev. Citol., 96, 121-155.
71.Jacobson, M. (1991) Developmental
Neurobiology, Plenum Press, N. Y.
72.Jackson, M. C., Scollard, D. M., Randall, J. M.,
and Lenney, J. F. (1994) Brain Res. Bull., 33,
379-385.
73.McFarland, G. A., and Holliday, R. (1999) Exp.
Gerontol., 34, 35-45.
74.Boldyrev, A. A., Johnson, P., Wei, Y., Tan, Y.,
and Carpenter, D. O. (1999) Neurosci. Lett., 263,
169-172.
75.Preston, J. E., Hipkiss, A. R., Himsworth, D. T.
J., Romero, I. A., and Abbott, J. N. (1998) Neurosci. Lett.,
242, 105-108.
76.Bakardjiev, A. (1998) Glia, 24,
346-351.
77.Kastritsis, C. H., and McCarthy, K. D. (1993)
Glia, 8, 106-113.
78.Berger, T., Muller, T., and Kettenmann, H. (1994)
Persp. Develop. Neurobiol., 2, 347-356.
79.Carmignoto, G., Pasti, L., and Pozzan, T. (1998)
J. Neurosci., 18, 4637-4645.
80.Bezzi, P., Carmignoto, G., Pasti, L., Vesce, S.,
Rossi, D., Rizzini, B. L., Pozzan, T., and Volterra, A. (1998)
Nature, 391, 281-285.
81.Araque, A., Parpura, V., Sanzgiri, R. P., and
Haydon, P. G. (1999) TINS, 22, 208-215.
82.Graziadei, P. P. C., and Monti Graziadei, G. A.
(1978) Neuronal Plasticity (Cotman, C. W., ed.) Raven Press, N.
Y., pp. 131-153.