Carnosine as a Potential Anti-senescence Drug
S. Gallant1, M. Semyonova2, and M. Yuneva3*
1Zoetic Neurosciences Ltd., 4 Ivester Court, Wing Road, Leighton Buzzard, Beds, LU7 7NW, Great Britain; fax: 44-1525-85-0109; E-mail: steven.gallant@dial.pipex.com2Department of Embriology, School of Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; E-mail: msemenova@soil.msu.ru
3International Center for Biotechnology and Center of Molecular Medicine, Lomonosov Moscow State University (Department of Biochemistry), Moscow, 119899 Russia; fax: (095) 939-1398; E-mail: MYuneva@genebee.msu.su
* To whom correspondence should be addressed.
Received December 20, 1999
The naturally occurring dipeptide carnosine (beta-alanyl-L-histidine) has been found to exert an anti-senescence effect when used as a dietary supplement. Carnosine clearly improved the external appearance of experimental animals and provided beneficial physiological effects, thus maintaining the animals in better condition than control animals receiving no carnosine or a mixture of beta-alanine and L-histidine.
KEY WORDS: aging, ROS, anti-senescence factors, carnosine, protein glycation
Many diverse properties of the dipeptide carnosine, which are more completely described elsewhere in this volume, stimulated the idea that carnosine may have some useful therapeutic value, particularly with regard to old age. We were greatly inspired by the pioneering work of McFarland and Holliday [1], where it was shown and later confirmed [2] that the endogenous dipeptide carnosine (beta-alanyl-L-histidine) was able not only to rejuvenate human cells in cultures, but also influence the formation of long-lived clones “affecting earlier events during serial subculture”. The work of Kantha et al. who had also shown an anti-senescence effect of carnosine in vitro [3] encouraged us to test it on small mammals in order to obtain some in vivo data. The Senescence Accelerated Mice line (hereafter SAM) was chosen for our initial experiments because of the short lifespan of the animals and the already large amount of literature that exists about this line [4]. In SAM animals, an over-production of free radicals occurring in their tissues may cause the accelerated senescence [5-8]. The full details of our experiments are described elsewhere [9, 10]. These experiments revealed that carnosine at a daily dose of 100 mg/kg of body weight was able to extend the mean lifespan of the mice by 20% but had no effect on the maximum lifespan.
The polypotent effects of carnosine which are described throughout this journal and the wider literature make it an ideal candidate as a so-called “geroprotector”--an agent which may delay or prevent some conditions intrinsic with old age.
EXPERIMENTAL METHODS
The animals of SAMR1 (resistant) and SAMP1 (prone) strains were kept under standard conditions on a balanced diet. Aging features were found to accumulate at the age of 10 months for the SAMP1 strain in comparison with SAMR1 animals of the same age. This was particularly reflected in the external appearance of SAMP1 and in their behavioral reactions [9]. The maximum lifespan of SAMP1 is 15 months and for SAMR1 it is 24 months. At the age of 2 months, mice of SAMP1 strain of both sexes were randomly divided into three groups of 80 animals each. One experimental group received carnosine in their drinking water in an amount corresponding to 100 mg/kg of body weight per day. Another experimental group received the mixture of beta-alanine and L-histidine (the compounds were mixed in molar proportion as they are in carnosine). In order to determine behavior and exterior characteristics of the animals, the Grading Score System (GSS) [4] was used.
RESULTS AND DISCUSSION
Aging and dependent pathologies are supposed to be connected with increased production of reactive oxygen species (ROS) [11, 12]. Consequently, the use of ROS scavengers should have been logical for treatment. Indeed, many synthetic ROS scavengers like N-tert-butyl-alpha-phenylnitrone [13] and the lazaroids [14, 15] are under clinical evaluation as stroke therapies. However, many have significant side effects and as such, severe clinical limitations. Generally, synthetic ROS scavengers have sufficient ability to protect the brain but are generally accompanied by unacceptable side effects or other limitations arising from their xenobiotic nature. For this reason a search had been going on for some time to find a natural brain protector that may be used not only in an emergency situation, but also on a prophylactic basis. Carnosine could be one of these natural brain protectors [16, 17]. Our experiments with SAMP1 may also support this possibility.
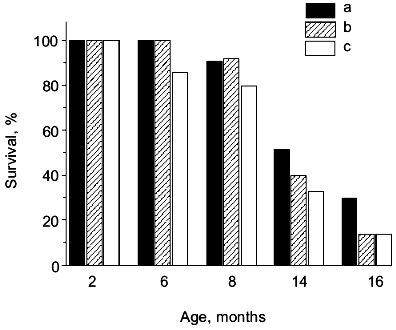
If SAMP1 animals were maintained on a carnosine-containing diet, their maximum lifespan hardly increased, while the mean lifespan was 20% longer, accompanied by an increased number of animals living to old ages (figure). Additionally, the exterior of the SAMP1 animals treated with carnosine was much more satisfactory. According to the GSS parameters, these animals may be said to have become more resistant to aging (table). With regard to certain parameters there were no differences found between SAMP1 treated and non-treated with carnosine. However, some others, such as periophtalmic lesions (p = 0.001), glossiness of the skin (p = 0.001), physiological reactivity (p = 0.001), skin ulcers (p = 0.001) and spinal lordokyphosis (p = 0.02), showed a strong protective effect of carnosine against aging. The body weight of the animals was controlled, so we cannot say dietary restriction played a role in the carnosine effect.
Morphological and physiological characteristics of 10-month-old SAMP1 mice (n = 36) treated and non-treated with carnosine (criteria used are from the GSS (Grading Score System) representative analysis [4]; % of animals in groups corresponding to each criterion is presented; data are mean ± S.E.M.)The mean lifespan of SAMP1 animals treated with carnosine (a) in comparison with the mean lifespan of animals treated with the mixture of beta-alanine and L-histidine (b) and of control animals (no additions) (c). For groups (a) and (c) the differences are significant for ages 6, 8, 14, and 16 months, while for groups (a) and (b) the data are statistically significant only for ages 14 and 16 months.
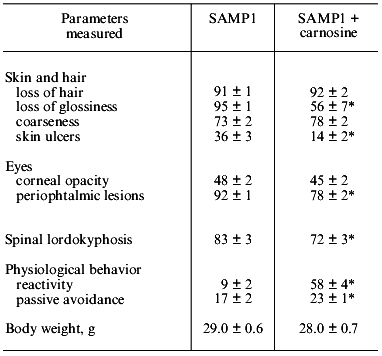
*p < 0.05 (SAMP1 versus SAMP1 + carnosine).
Some biochemical characteristics of SAMP1 animals were also affected under carnosine treatment, which was shown in our previously published experiments [10]. The brain membranes of animals treated with carnosine were characterized by a lower malonic dialdehyde (MDA) level, both initial (decreased by 35%) and that produced at 90 min of lipid peroxidation induced by ferric ions and ascorbic acid (by 30%) [10]. Comparison of brain mitochondrial monoamine oxidase B (MAO B) showed a 44% decrease in its activity for carnosine treated mice, which may indicate the creation of conditions where fewer toxic compounds would be produced in brain tissue [18].
However, these facts may not be the entire explanation of the effect of carnosine as other ROS scavengers have failed to rejuvenate human fibroblasts [1]. Some experiments carried out in different laboratories demonstrate that one of carnosine's targets can be DNA molecules. Ikeda and co-authors showed that carnosine caused expression of vimentin protein in rat 3Y1 fibroblasts, resulting in extending cell viability (vimentin “is thought to play a fundamental role in maintaining cell structure and integrity”) [19]. In the experiments of Gille et al., carnosine significantly reduced the frequency of aberrant cells and number of chromosomal aberrations in Chinese hamster cell cultures exposed to hyperoxia [20]. In our experiments glutamate receptors of N-methyl-D-aspartate (NMDA) type were characterized by higher level of ligand binding in brain of mice treated with carnosine (this fact corresponds to positive changes in the behavior of this group of animals) (table) [10]. These facts suggest that carnosine's effect can be also aimed at DNA, influencing the expression of certain genes.
Moreover, A. Hipkiss describes in this volume how carnosine, being a potent antiglycating agent, may suppress the deleterious effects of protein carbonyls by reacting with them to form adducts, which may either prevent their interaction with scavenging AGE (Advanced Glycated End Products) “receptors” (RAGES), or alter the cellular response by modulating signal transduction and subsequent generation of ROS.
It is known that carnosine is metabolized into beta-alanine and L-histidine and as they are both biologically active molecules, they could have played some role in the anti-senescence effect of carnosine in SAM. However, according to data obtained in our experiments we observed no effect of these compounds on the mean lifespan when animals were treated with the mixture of beta-alanine and L-histidine, taken in the same molar proportion as they are in carnosine (figure). This treatment also had no influence on the exterior of the animals, unlike the effect carnosine produced. Analysis of these data shows that carnosine acts as a true antioxidant protector rather than as an anabolic drug; the weight of the animals treated with carnosine was not significantly different from that of the control animals (table).
Anyway, the question “How could such a small molecule have such profound effects?” remains unanswered, though we hope through increased global scientific collaboration that we shall have the answers sooner rather than later.
The authors are particularly grateful to Professor A. Boldyrev who has supervised this entire anti-senescence project and devoted much time and energy to it. We are also grateful to all the staff of Moscow State University and the Institute of Neurology (Russian Academy Medical Sciences, Moscow) who worked on this project. Steven Gallant wishes to personally thank Paul Michaels for his help and encouragement at the beginning of the project. We are further grateful to all those who took part in this project but are too numerous to name.
The study was supported by the Russian Foundation for Basic Research (grant No. 00-04-48767).
REFERENCES
1.McFarland, G. A., and Holliday, R. (1994) Exp.
Cell Res., 212, 167-175.
2.McFarland, G. A., and Holliday, R. (1999) Exp.
Geront., 34, 35-45.
3.Kantha, S. S., Wada, S., Tanaka, H., Takeuchi, M.,
Watabe, S., and Ochi, H. (1996) Biochem. Biophys. Res. Commun.,
223, 278-282.
4.Takeda, T., Hosokawa, M., and Higuchi, K. (1991)
J. Am. Geriatrics Soc., 39, 911-919.
5.Ferrandiz, M. L., Martinez, M., Juan, E., Diez, A.,
Bustos, G., and Miquel, J. (1994) Brain Res., 644,
335-338.
6.Nishikawa, T., Takahashi, J. A., Fujibayashi, Y.,
Fujisawa, H., Zhu, B., Nishimura, Y., Ohnishi, K., Higuchi, K.,
Hashimoto, N., and Hosokawa, M. (1998) Neurosci. Lett.,
254, 69-72.
7.Park, J. W., Choi, C. H., Kim, M. S., and Chung, M.
H. (1996) J. Gerontol., 51, B337-B345.
8.Yokoi, I., Inada, K., Habu, H., Kabuto, H., Mori,
A., Ohyama, H., Iwaya, K., Koyama, S., Nishijima, Y., and Nishijima, K.
(1995) in Oxidative Stress and Aging (Cutler, R. G., Packer, L.,
and Mori, A., eds.) Berkhauser Verlag, Basel, pp. 387-393.
9.Bulygina, E., Gallant, S., Kramarenko, G.,
Stvolinsky, S., Yuneva, M., and Boldyrev, A. (1999) J. Anti-Aging
Med., 2, 43-49.
10.Yuneva, M. O., Bulygina, E. R., Gallant, S. C.,
Kramarenko, G. G., Stvolinsky, S. L., Semyonova, M. L., and Boldyrev,
A. A. (1999) J. Anti-Aging Med., 2, 337-342.
11.Carney, J. M., and Carney, A. M. (1994) Life
Sci., 55, 2097-2103.
12.Koltover, V. K. (1998) Adv. Gerontol.,
2, 37-41.
13.Butterfield, D. A., Koppal, T., Howard, B.,
Subramaniam, R., Hall, N., Hensley, K., Yatin, S., Allen, K., Aksenov,
M., Aksenova, M., and Carney, J. (1998) Ann. N. Y. Acad. Sci.,
854, 448-462.
14.Jellinger, K. A. (1999) Drugs Aging,
14, 115-140.
15.Taylor, B. M., Fleming, W. E., Benjamin, C. W.,
Wu, Y., Mathews, W. R., and Sun, F. F. (1999) J. Pharmacol. Exp.
Ther., 276, 1224-1231.
16.Boldyrev, A. A., Stvolinsky, S. L., Tyulina, O.
V., Koshelev, V. B., Hori, N., and Carpenter, D. (1997) Cell. Molec.
Neurobiol., 17, 259-271.
17.Boldyrev, A. A. (1998) Carnosine [in
Russian], Moscow University Publishing House, Moscow, p. 320.
18.Burchinsky, S. G., and Kuznecova, S. M. (1988)
Voprosy Med. Khim., 34, 2-9.
19.Ikeda, D., Wada, S., Yoneda, C., Abe, H., and
Watabe, S. (1999) Cell Struct. Funct., 24, 79-87.
20.Gille, J. J. P., Pasman, P., Berkel, C. G. M.,
and Joenje, H. (1991) Mutagenesis, 6, 313-318.