Luteinizing Hormone-Releasing Hormone in Thymus and Hypothalamus of Rat Fetuses: Suppressing Effect of Antagonist and of Antibodies on Concanavalin A-Induced Proliferation of Thymocytes
L. A. Zakharova1*, I. V. Malyukova1, E. I. Adamskaya2, T. A. Kuznetsova2, and I. V. Shishkina2
1Kol'tsov Institute of Developmental Biology, Russian Academy of Sciences, ul. Vavilova 26, Moscow, 117871 Russia; fax: (095) 135-8012; E-mail: zaharova@ibrran.msk.su2Endocrinology Research Center, Russian Academy of Medical Sciences, ul. Dmitriya Ul'yanova 11, Moscow, 117036 Russia
* To whom correspondence should be addressed.
Received March 9, 2000; Revision received May 10, 2000
The effect of endogenous luteinizing hormone-releasing hormone (LHRH) on the proliferation induced by concanavalin A (Con A) in rat fetal thymocytes was studied. A selective antagonist (2 µg per fetus) or antibodies to LHRH (20 µl per fetus) were injected in utero into 20-day-old rat fetuses, and this resulted in a two- or fivefold decrease in the Con A-induced proliferation of thymocytes, respectively. In combined culture of the antagonist (10-5-10-6 M) with fetal thymocytes, the proliferative response was not decreased. The concentration of LHRH was determined by radioimmunoassay in tissues of immunocompetent organs and in blood serum of 18- and 21-day-old fetuses, and the hormone was found in the hypothalamus, thymus, and peripheral blood. The initially low level of LHRH in the thymus increased by 65 and 40%, respectively, on the first day after birth and became similar to the level in the hypothalamus. In the fetal blood serum, the LHRH level was significantly higher than in the thymus and hypothalamus of fetuses of the same age. The hormone concentration was greatest in the 18-day-old fetuses, and it decreased twofold by the 21st day. The findings indicate that LHRH is involved in regulation of T-cell immunity even during prenatal ontogenesis.
KEY WORDS: LHRH, antibodies, thymus, hypothalamus, radioimmunoassay, proliferative response, prenatal ontogenesis
Abbreviations: LHRH) luteinizing hormone-releasing hormone; Con A) concanavalin A; anta-LHRH) antagonist [D-pGlu-D-Phe-D-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2]; PBS) phosphate-buffered saline.
Experimental data have recently appeared indicating that LHRH is
involved in the differentiation of lymphocytes and regulation of
humoral and cellular immunity in man and animals during postnatal
ontogenesis [1-3]. In addition
to the hypothalamus-hypophysis system, LHRH and its receptors are also
synthesized in the immune system tissues such as thymus, spleen, and
peripheral blood lymphocytes [4-8]. Therefore, LHRH is suggested to have not only a
central effect but to be also involved in autocrine or paracrine
regulation of the immune response. However, there are virtually no data
on the role of LHRH in the development of immunity during prenatal
ontogenesis. We have shown earlier that the systemic injection of LHRH
into hypothalamectomized in utero rat fetuses restored the
suppressed mitogen-induced proliferation of the thymocytes [9, 10].
This work was designed to study the effect of an antagonist of LHRH receptors and of antibodies to LHRH on thymocyte proliferation induced by concanavalin A and also to determine the level of this hormone in the thymus, liver, hypothalamus, and blood serum of rat fetuses.
MATERIALS AND METHODS
Pregnant Wistar rats were used. The pregnant rats were maintained under standard conditions with controlled illumination from 5 am to 7 pm. Twenty-day-old pregnancy fetuses from one uterine horn were injected intraperitoneally with rabbit antibodies to LHRH (Sigma, USA, catalog No. L 8391). The lyophilized highly specific rabbit antiserum was diluted with 0.5 ml of 0.9% NaCl solution and injected in a volume of 20 µl. Fetuses from the other uterine horn were concurrently injected with 20 µl of 0.9% NaCl solution or with normal rabbit serum. Moreover, a highly specific antiserum was used that was prepared in the Laboratory of Endocrine System Physiology (Endocrinology Research Center, Russian Academy of Medical Sciences) by immunization of rats with a conjugate of LHRH (Sigma) with bovine serum albumin (BSA, Sigma) [11]. The antiserum at final dilution 1 : 10,000 provided high-specificity radioimmunoassay. In some experiments, 20-day-old fetuses from one uterine horn were injected intraperitoneally with the selective antagonist [D-pGlu-D-Phe-D-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH2] (anta-LHRH, Sigma) at the dose 2 or 20 µg per fetus in 20 µl of 0.9% NaCl solution, and fetuses of the other horn were injected with 20 µl of the solvent. Every experimental and control group included 5-8 fetuses from at least two pregnant rats.
The LHRH level was determined in tissues of thymus, liver, and hypothalamus and also in blood serum of 18- and 21-day-old fetuses, 3-day-old rat pups, and of the pregnant females at the corresponding times. The fetuses were isolated from the uterus under pentobarbital anesthesia. After differentiating by sex, the thymus, liver, and hypothalamus including the anterior and mediobasal parts were isolated (for every determination, material from 4-5 fetuses were combined per sample), homogenized with 10 volumes of 0.2 M CH3COOH, heated for 10 min in a water bath at 100°C, then cooled to 0°C, and the supernatant was separated by centrifugation for 15 min at 1000g. Blood was collected from 18- and 21-day-old fetuses (samples from 6-8 fetuses were combined), from 3-day-old rat pups (samples from 2-3 animals were combined), and from the pregnant females. The serum was separated by centrifugation (30 min at 400g) after the clotting of the blood. The resulting supernatant and blood serum were frozen with liquid nitrogen and stored at -70°C.
The level of LHRH in the tissues and blood serum was determined by a slightly modified [11] radioimmunoassay using double antibodies [12]. Plastic tubes were supplemented successively with: 200 µl of 0.01 M phosphate-buffered saline (PBS) containing 1% BSA and 0.1% Triton X-100 (pH 7.4), 100 µl of rabbit antibodies to LHRH diluted with PBS containing 3% normal rabbit serum (to assess the nonspecific binding, the antibodies to LHRH were not introduced), 100 µl of synthetic LHRH (Sigma) at varied concentrations (from 1.8 to 1000 pg per tube), or the supernatant from the rat tissues or the rat blood serum. The reagents were mixed, incubated for 16 h at 4°C,then 125I-labeled LHRH was added into all tubes and the incubation was continued for 24 h at 4°C. To separate the bound LHRH from the unbound hormone, 100 µl of sheep antibodies to rabbit gamma-globulin (Gamaleya Institute of Experimental Medicine, Moscow) pre-diluted tenfold with PBS and 500 µl of 6% polyethylene glycol 6000 (Ferak, Germany) were added into all tubes, and the tubes were centrifuged at 1500g for 20 min at 4°C.
LHRH was labeled with Na125I using iodogen (Serva, Germany) [13]. Chloroform (80 µl) with 3 µg iodogen was added into glass tubes, then evaporated under nitrogen until the chloroform was removed completely, and then the tubes were supplemented with Na125I (0.5 mCi), 25 µl of 0.05 M PBS (pH 7.4), and 5 µl of LHRH (Sigma) solution in 0.01 M CH3COOH (1 µg.µl). The mixture was incubated for 10 min at 0°C, placed onto a column (1 ×_12 cm) with Sephadex G-25 fine (Pharmacia, Sweden) equilibrated with 0.01 M CH3COOH containing 0.1% BSA, and eluted with the same solution. The specific radioactivity of the labeled peptide was 160-200 µCi.µg, consistent with the literature data [14]. The radioactivity of samples was determined with a gamma-spectrometer (Searle, The Netherlands) with a counting efficiency of 90%. Variation coefficients within and between the experiments were 7 and 15%, respectively.
Functional activity of the T-system of immunity in 21- and 22-day-old rat fetuses was assessed by the thymocyte proliferation induced in vitro by Con A (2.5 µg/ml). The fetal thymocytes (2·105 cells/well in 200 µl of medium) were incubated in 96-well plastic plates (Costar, USA) in the RPMI-1640 medium (Serva) with 10% bovine serum (Gibco, Great Britain), 2 mM L-glutamine (ICN, Great Britain), 10 mM HEPES (Serva), and gentamicin (50 µg/ml, Gibco) for 72 h in the presence of 5% CO2 at 37°C. For the last 18 h of the incubation, [3H]thymidine (60 Ci/mmole, Amersham, Great Britain) was added at 0.5 µCi per well. In some in vitro experiments, the Con A-activated thymocytes were supplemented with LHRH at the doses 10-5 and 10-6 M at the start of incubation. Five wells were used for every experimental point. The cell viability tested with Trypan Blue was higher than 95%.
The data were processed using Student's t-test.
RESULTS
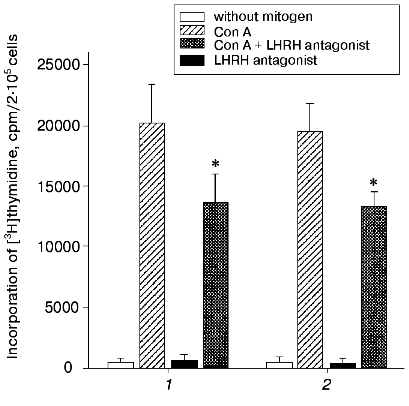
Anta-LHRH was injected in utero into 20-day-old rat fetuses, and its effect on the proliferation of Con A-activated thymocytes was determined 48 h later. Anta-LHRH (2 and 20 µg per fetus) caused a 40-50% decrease in the thymocyte proliferation compared to the control fetuses injected with 0.9% NaCl solution (Fig. 1). Anta-LHRH had no effect on proliferation of thymocytes that were not activated with Con A (Fig. 1).
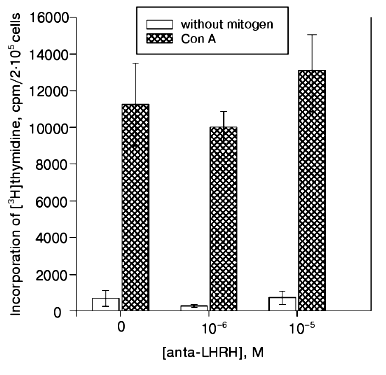
The Con A-induced proliferation response in the culture of thymocytes of 22-day-old fetuses did not decrease in the presence of anta-LHRH (10-5 and 10-6 M) (Fig. 2).Fig. 1. The in vivo effect of LHRH antagonist on Con A-induced thymocyte proliferation of rat fetuses. The antagonist was injected in utero into 20-day-old fetuses in one uterine horn at the dose 2 µg/fetus (1) or 20 µg/fetus (2) in 20 µl of 0.9% NaCl solution, and fetuses in the other horn were injected with 20 µl of 0.9% NaCl solution. The proliferative activity of the thymocytes was assessed on the 22nd day of prenatal development. The thymocytes were incubated for 72 h; for the last 18 h they were cultured in the presence of [3H]thymidine (60 Ci/mmole) added at 0.5 µCi/well. The results are presented as the means ± the standard error (n = 5), * p < 0.05 (Student's t-test).
In a series of experiments, the effect of anta-LHRH on the Con A-induced thymocyte proliferation was studied in males and females. The proliferation in males and females was virtually the same in both the control (19,900 ± 2,100 and 20,000 ± 2,400 cpm, respectively, n = 15) and after the injection of fetuses with anta-LHRH (12,100 ± 1,500 and 11,800 ± 1,200 cpm).Fig. 2. The in vitro effect of LHRH antagonist (anta-LHRH) on the Con A-induced thymocyte proliferation of rat fetuses. The proliferative activity of the thymocytes was assessed on the 22nd day of prenatal development. LHRH antagonist was added at the doses 10-5 and 10-6 M to the Con A-activated thymocytes at the start of incubation. The results are presented as the means ± the standard error (n = 3).
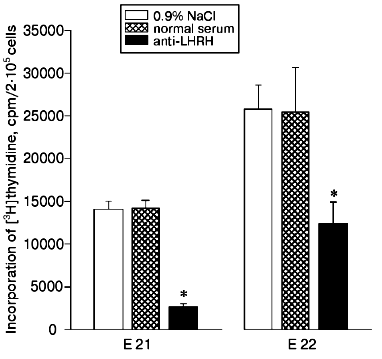
The in utero injection of antibodies to LHRH (20 µl per fetus) into the 20-day-old rat fetuses also decreased the proliferation of thymocytes induced by Con A compared to the control fetuses injected with the solvent or with normal rabbit serum (Fig. 3). Twenty-four hours after the injection of the antibodies (on the 21st day of prenatal development), the proliferative immune response was suppressed fivefold, whereas on the 22nd day the suppression was twofold, and this is suggested to be due to a decrease in the amount of the antibodies by the later time.
The presence of LHRH was detected by radioimmunoassay in the thymus of rat fetuses. During the first days after birth, the hormone content in the thymus was increased about 65 and about 40% compared to the 18- and 21-day-old fetuses, respectively (table). The concentration of LHRH in the fetal thymus was 30-35% lower than in the hypothalamus and reached the same level in the newborn rats. The LHRH contents in the studied tissues were similar in males and females. In the liver of fetuses and newborn rats, LHRH was virtually not found (table).Fig. 3. Effect of antibodies to LHRH on thymocyte proliferation induced by Con A in rat fetuses. Rabbit antibodies to LHRH in 20 µl of 0.9% NaCl solution were injected in utero into the 20-day-old fetuses in one uterine horn. Concurrently, the fetuses of the other horn were injected with 20 µl of 0.9% NaCl solution or with normal rabbit serum. The proliferative activity of the thymocytes was assessed on the 21st (E 21) and on the 22nd (E 22) days of prenatal development. The results are presented as means ± standard error (n = 8), * p < 0.01.
Concentration of LHRH (pg.µg protein) in the thymus, liver, and
hypothalamus of rat fetuses and newborn rats
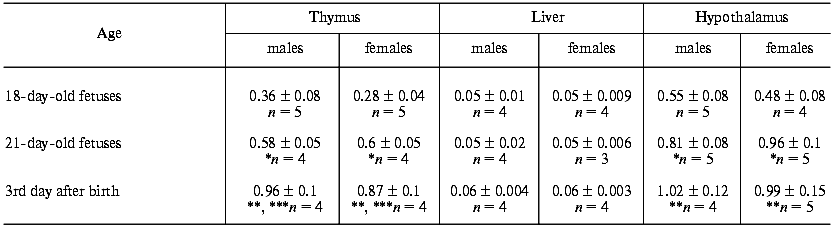
Note: Results are presented as means ± standard error.
*p < 0.05 compared to 18-day-old fetuses.
**p < 0.01 compared to 18-day-old fetuses.
***p < 0.005 compared to 21-day-old fetuses.
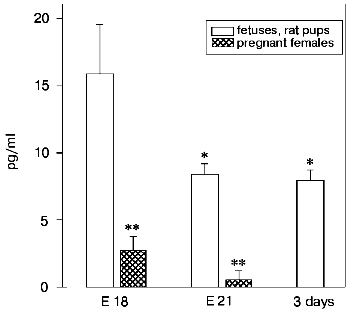
A significant content of LHRH was also found in blood serum of fetuses compared to its content in the thymus and hypothalamus of the same fetuses and to the blood serum of their mothers (Fig. 4). The highest concentration of LHRH was found in the 18-day-old fetuses; then it decreased about twofold in the 21-day-old fetuses and after birth.
Fig. 4. Concentration of LHRH (pg/ml) in blood serum of fetuses and of newborn rats. The concentration of LHRH was determined on the 18th (E 18, n = 15) and on the 21st (E 21, n = 7) prenatal day, on the 3rd day after birth (n = 5), and in females on the 18th and on the 21th days of pregnancy (n = 15 and n = 7, respectively). The results are presented as means ± standard error; * p < 0.05 compared to 18-day-old fetuses.
DISCUSSION
The in utero injection of anta-LHRH and of antibodies to LHRH into 20-day-old rat fetuses suppressed the Con A-activated proliferation of thymocytes (Figs. 1 and 3). These data are consistent with our earlier findings that encephaloectomy of fetuses in utero also suppressed by 30-40% the proliferative immune response of thymocytes, whereas the injection of LHRH both in vivo and in vitro restored it completely [10].
LHRH seems to be involved in the formation of the immune system during early stages of its development. Thus, the injection of anta-LHRH into monkeys within the first days after birth decreased the numbers of mature T- and B-lymphocytes in the thymus and spleen [15] and also the number of circulating lymphocytes that were observed up to 5 years of age [16].
High doses of LHRH suppressed the differentiation of precursors of T- and B-lymphocytes in the primary lymphoid tissues, and this resulted in their decreased numbers in the secondary lymphoid tissues [2].
Some direct and indirect mechanisms of the effect of LHRH on the immune system are suggested. The hormone is suggested to interact with specific receptors on the thymus epithelial cells that should induce them to produce thymic peptides involved in the maturation of T-lymphocytes [3]. LHRH can also interact immediately with receptors located on lymphocytes [1, 4, 17, 18] and regulate their level [7]. Moreover, LHRH seems to be involved in lymphocyte activation and induce the expression of receptors to interleukin-2 (IL-2) [19]. LHRH and its agonists were recently shown to stimulate a dose-dependent synthesis of mRNA of IL-2 gamma-chain [8] and also the synthesis of gamma-interferon, which is responsible for induction of IL-2 production in human peripheral blood lymphocytes [20].
It is known that LHRH is synthesized in the hypothalamus of rat fetuses even during early stages of development. Its level is increased by birth but remains relatively low [21]. According to our findings, a small amount of LHRH is also synthesized in the fetal thymus (table). LHRH synthesis in human and animal lymphocytes suggests the involvement of this hormone in the autocrine or paracrine regulation of the immune response. The leukemic cell line Jurkat similar in phenotype to human T-lymphocytes was shown to express mRNA of LHRH and to secrete LHRH and its precursor into the culture medium [5]. The proliferative activity of this cell line was increased by both endogenous and exogenous hormone, while the addition of anta-LHRH suppressed the cell proliferation.
It seems that LHRH from the fetal peripheral blood (its source is now under study) is also involved in the regulation of the proliferative immune response. We have shown that the content of LHRH in the blood of fetuses was significantly higher than in the thymus, hypothalamus, and blood serum of their mothers (table, Fig. 4). The highest blood level of LHRH in the 18-day-old fetuses is concurrent with the period of lymphocyte maturation in the immunocompetent organs [22]. However, the immunomodulating effect of LHRH within prenatal development is suggested to occur via the interaction between the immune and the hypothalamus-hypophysis systems. Marchetti suggested [23] that LHRH is a primary signal molecule in neuroendocrine-immune interaction. We have shown that, unlike the injection of anta-LHRH into fetuses, its presence in a culture of thymocytes for 3 days had no effect on the Con A-induced proliferation (Fig. 2). Moreover, in the case of simultaneous extirpation of the hypothalamus and hypophysis, the injection of exogenous LHRH partially restored the suppressed mitogen-dependent proliferation of thymocytes [10]. It is also likely that sex hormones, especially estrogens, after interaction with specific receptors, concurrently modulate the synthesis of thymulin and LHRH in the thymus and hypothalamus of rat fetuses [6, 24].
Thus, various mechanisms are suggested to be involved in the effect of LHRH on the immune system, and we are continuing our studies in this area. In summary, our findings suggest that LHRH is involved into the development of T-cell immunity during prenatal ontogenesis.
The authors are grateful to Dr. M. V. Ugryumov and to Dr. V. N. Babichev for discussion of the findings and also to E. V. Proshlyakova for her technical assistance during the work.
This work was supported by the Russian Foundation for Basic Research (grant No. 98-04-49182).
REFERENCES
1.Morale, M. C., Batticane, N., Bartoloni, G.,
Guarello, V., Farinella, Z., Galasso, M. G., and Marchetti, B. (1991)
Endocrinology, 128, 1073-1085.
2.Rao, L. V., Cleveland, R. P., Kimmel, R. J., and
Ataya, K. M. (1995) Am. J. Reprod. Immunol., 34,
257-266.
3.Fabris, N., Mocchegiani, E., and Provinciali, M.
(1997) Exp. Gerontol., 32, 415-429.
4.Weesner, G. D., Becker, B. A., and Matteri, R. L.
(1997) Life Sci., 61, 1643-1649.
5.Azad, N., LaPaglia, N., Kirsteins, L., Uddin, S.,
Steiner, J., Williams, D. W., Lawrence, A. M., and Emanuele, N. V.
(1997) J. Endocrinol., 153, 241-249.
6.Azad, N., LaPaglia, N., Agrawal, L., Steiner, J.,
Uddin, S., Williams, D. W., Lawrence, A. M., and Emanuele, N. V. (1998)
J. Endocrinol., 158, 229-235.
7.Jacobson, J. D., Crofford, L. J., Sun, L., and
Wilder, R. L. (1998) Neuroendocrinology, 67, 117-125.
8.Chen, H. F., Jeung, E. B., Stephenson, M., and
Leung, P. C. (1999) J. Clin. Endocrinol. Metab., 84,
743-750.
9.Zakharova, L. A., Malyukova, I. V., Sapronova, A.
Ya., Proshlyakova, E. V., Mel'nikova, V. I., and Ugryumov, M. V. (1995)
Dokl. Ros. Akad. Nauk,345, 830-832.
10.Zakharova, L. A., Potapova, A. A., Malyukova, I.
V., Proshlyakova, E. V., and Ugryumov, M. V. (1997) Dokl. Ros. Akad.
Nauk, 357, 269-271.
11.Samsonova, V. M., and Babichev, V. N. (1977)
Probl. Endokrinol., 23, 58-64.
12.Nett, T. M., Akbar, A. M., Niswender, G. D.,
Hedlund, M. T., and White, W. F. (1973) J. Clin. Endocrinol.
Metab., 36, 880-887.
13.Regoeczi, E. (1983) Int. J. Peptide Protein
Res., 22, 422-433.
14.Bourguignon, J.-P., Gerard, A., and Franchimont,
P. (1984) Neuroendocrinology, 38, 376-381.
15.Mann, D. R., Howie, S., Paulsen, D. F., Akinbami,
M. A., Lunn, S. F., and Fraser, H. M. (1998) Am. J. Reprod.
Immunol., 39, 256-265.
16.Gould, K. G., Akinbami, M. A., and Mann, D. R.
(1998) Dev. Comp. Immunol., 22, 457-467.
17.Costa, O., Mulchahey, J., and Blalock, J. E.
(1990) Progr. Neuroendocrinol. Immunol., 3, 35-60.
18.Adams, B. M., Sakurai, H., and Adams, T. E.
(1997) J. Reprod. Fertil., 111, 207-212.
19.Batticane, N., Morale, M. C., Gallo, F.,
Farinella, Z., and Marcetti, B. (1991) Endocrinology,
129, 277-286.
20.Grasso, G., Massai, L., De Leo, V., and
Muscettola, M. (1998) Life Sci., 62, 2005-2014.
21.Aubert, M. L., Begeot, M., and Winiger, B. P.
(1985) Endocrinology, 116, 1565-1676.
22.Van Rees, E. P., and Sminia, T. (1990) Dev.
Comp. Immunol., 14, 9-18.
23.Marchetti, B., Gallo, F., Farinella, Z., Tirolo,
C., Testa, N., Romeo, C., and Morale, M. C. (1998) Ann. N. Y. Acad.
Sci., 1, 205-248.
24.Sakabe, K., Seiki, K., Sakai, N., and He, W.
(1996) Med. Sci. Res., 24, 439-442.