Antigen-Binding Activity of Monoclonal Antibodies after Incubation with Organic Solvents
Ya. I. Melnikova, S. G. Odintsov, Z. I. Kravchuk, and S. P. Martsev*
Institute of Bioorganic Chemistry, National Academy of Sciences of Belarus, ul. Akademika Kuprevicha 5/2, Minsk, 220141 Belarus; fax: +375-17-263-7274; E-mail: martsev@ns.iboch.ac.by* To whom correspondence should be addressed.
Received May 17, 2000
Effects of four organic solvents--methanol, trifluoroethanol, dimethylsulfoxide, and dimethylformamide (DMF)--on the ferritin-binding activity of three monoclonal mouse antibodies of IgG2a and IgG1 subclasses were studied. The ferritin-binding constants of monoclonal antibodies G10 and F11 (the IgG2a subclass) were increased 2-6-fold after incubation with DMF and removal of the organic solvent by gel filtration. The maximum effect on the F11 antibodies was found in the presence of 5-13% DMF and on the G10 antibodies at 11-40% DMF. The effect remained after the removal of DMF from the incubation medium, and this suggests that the incubation with DMF resulted in irreversible conformational changes of the antibodies and in production of active conformers of the G10 and F11 antibodies. These conformations occurred within 15-60 min. The long-term stability and the fluorescence of the antibodies exposed to DMF suggest that the conformational changes were not global, but involved small and relatively independent structural elements of the antibodies, either of hypervariable CDR loops in variable domains or of the hinge region of the antibodies. The affinity of the C5 antibodies of the mouse IgG1 subclass was decreased after incubation with DMF. The activation was a solvent-specific effect because incubation of the G10 antibodies with methanol and dimethylsulfoxide decreased the affinity for the antigen, and incubation with trifluoroethanol virtually did not affect it. Relatively small changes in the antigen-binding activity of the antibodies were found even after the incubation with 5% organic solvent.
KEY WORDS: immunoglobulin, IgG2a, IgG1, antibody conformers, antibody modification, affinity of antibodies, intrinsic fluorescence, dimethylformamide
Changes in protein structure induced by chemical or physical agents are usually associated with a complete or partial loss of the biological activity. Data on functional activation of protein molecules after their structural perturbation are very scarce [1-5]. Functional activation of proteins was mainly observed by approaches of gene engineering [4, 6, 7]. Note that most methods for chemical modification of proteins require organic solvents to be added because of the hydrophobicity of many chemical modifiers. The addition of small quantities of organic solvents is usually presumed to have no effect on both the conformation and function of a protein. However, our preliminary findings suggest that this presumption cannot be generalized.
Changes in properties of proteins in water-organic media are induced by changes in the properties of water at high and low concentrations of organic solvents due to changes in the system of hydrogen bonds and hydrophobic interactions in protein molecules [8-13]. To provide stabilization of a protein molecule, the ratio of hydrophobic and hydrophilic regions on its surface should have a certain value that depends on the protein structure. Changes in this ratio cause a rearrangement of hydrogen bonds that results in conformational changes of the whole molecule [14-17]. Some organic solvents were used earlier to induce in protein molecules conformations that were different from the native ones and had specific structural parameters. Trifluoroethanol was used to induce formation of alpha-helices in various proteins including such proteins with beta-sheet secondary structure as beta-lactoglobulin [18], the cardiotoxin III analog [10], ubiquitin [19], interleukin-1 [10, 20, 21], porin [22], and tumor necrosis factor [10]. The alcohol-induced denaturation of some proteins, e.g., cytochrome c, occurs through two steps and results in the appearance of a partially structured state resembling the “molten globule” [23-25]. An essentially disordered conformation of certain peptides, such as the atrial natriuretic factor or the brain natriuretic peptide, is converted to the more regulated state containing beta-structures on addition of hexafluoro-2-propanol into the incubation medium [26]. An addition of dimethylsulfoxide into the medium to provide the renaturation of lysozyme increases the rate of production of native-like structures and formation of the final native state [27].
In the present work, effects of relatively low concentrations of four organic solvents on the antigen binding by antibodies of the mouse IgG1 and IgG2a subclasses were studied. The organic solvents at the concentrations employed in the study did not induce global changes in the protein conformation but resulted in production of stable antibody conformers with changed functional activity. The organic solvents chosen, such as dimethylformamide (DMF), dimethylsulfoxide, and methanol, are generally used during chemical modification of proteins to dissolve the modifying agents [3, 5].
MATERIALS AND METHODS
The following reagents were used: caprylic acid (Fluka, Switzerland); trifluoroethanol (Wako Pure Chemical Industries Ltd, Japan); Sephacryl S-200 (Pharmacia, Sweden); Coomassie R-250 (Ferak, Germany); streptavidin conjugated with horseradish peroxidase, succinimide biotin ester (Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow); the conjugate of rabbit monospecific antibodies to mouse IgG with horseradish peroxidase (Research Institute of Especially Pure Biopreparations, St. Petersburg). Other reagents were of chemical purity, domestic production.
We have studied three preparations of antibodies to human spleen ferritin. Mouse monoclonal antibodies G10, F11 (the IgG2a subclass), and C5 (the IgG1 subclass) were prepared and characterized as described earlier [28, 29]. Ferritin was isolated from a human spleen samples and characterized by a conventional method [30].
The ferritin-specific monoclonal antibodies were conjugated with biotin by incubation of their solution (400 µg in 0.5 ml of 0.1 M NaHCO3, pH 8.5) with solution of N-hydrosuccinimide biotin ester (180 µg) in DMF (50 µl). The molar ratio modifier/protein was 120 : 1. After incubation for 1 h at room temperature, the reaction mixture was dialyzed overnight against 0.1 M sodium borate buffer (pH 8.5) at 4°C.
The monoclonal antibodies were immobilized on polystyrene plates (Nunc, Denmark; Linbro, USA) as described earlier [28].
The monospecific rabbit IgG was prepared by affinity chromatography on the immobilized ferritin [29]. Conjugates of the affinity-purified antibodies with horseradish peroxidase were obtained by a conventional method [30] using NaIO4 as oxidizer of the carbohydrate component of the peroxidase.
The monoclonal G10 antibodies were incubated with DMF as follows: tubes containing 300 µg of the antibodies in 0.1 M sodium borate buffer (pH 8.5) were supplemented with DMF to the final concentrations of 3, 5, 8, 11, 25, 40, and 50%, and the final volume of the mixture was 0.5 ml. After incubation for 1 h at room temperature, DMF was removed from the incubation medium by gel chromatography on a column (1 × 50 cm) with Sephacryl S-200 equilibrated with 0.1 M sodium borate (pH 8.5). The F11 and C5 antibodies were incubated in the presence of DMF concentrations from 3 to 50% and from 5 to 25%, respectively.
To determine the time course of conformational changes in the antibodies induced by DMF, the most effective concentration of the solvent was used: 25% for the G10 antibodies and 5% for the F11 antibodies. The organic solvent was removed as described above. The G10 antibodies were incubated with methanol, dimethylsulfoxide, and trifluoroethanol as described above, at the final concentrations of these organic solvents in the incubation medium 5, 10, and 20%, respectively.
The ferritin-binding constant of the G10 antibody interaction with
ferritin was determined by the method described in [31], which provides an adequate comparison of the
antibody binding constants for the soluble and immobilized forms of the
antigen. During the first stage of the analysis, increasing quantities
(from 25 to 1000 ng per sample) of the G10 antibodies, either native or
incubated with DMF, were incubated for 1.5 h with ferritin immobilized
in polystyrene plate wells. The immune complexes were quantified using
an anti-IgG-peroxidase conjugate (dilution 1 : 1000, incubation for 1.5
h). The constant of the antibody interaction with the immobilized
antigen was determined as the slope of the straight line in the
coordinate system as follows: along the ordinate axis the ratio of
absorption values A492 to the antibody concentration
in the sample; along the abscissa axis the absorption values
A492. At the second stage of the analysis, the wells
with the pre-immobilized antigen were supplemented with increasing
quantities of ferritin (50-2000 ng) and with the G10 antibodies (50 ng)
in 0.2 ml of 0.05 M sodium phosphate buffer (pH 7.4) containing 1% BSA
and 0.2 M NaCl and incubated for 1.5 h. Then the plates were washed and
the resulting immune complexes were developed with the
anti-IgG-peroxidase conjugate. The experimental data were presented as
the dependence of the binding ratio in the absence of a competing agent
(A0) to the binding in its presence (A) on the
concentration of the soluble antigen. In this coordinate system, values
of a parameter r were determined for each antigen preparation.
Values of the interaction constants of the G10 antigen with the soluble
ferritin were obtained using the formula:
The ferritin-binding constants of the native F11 antibodies and of those incubated with DMF were determined by competitive enzyme-linked immunoassay (ELISA) using ferritin immobilized in polystyrene plate wells, a conjugate of the monospecific rabbit IgG with horseradish peroxidase (6 ng), and increasing quantities of the native or DMF-incubated F11 antibodies. The monospecific rabbit IgG conjugate with horseradish peroxidase was used based on the effective competition of the monoclonal F11 antibodies and polyclonal rabbit monospecific antibodies: the polyclonal antibodies displaced up to 60% of the monoclonal antibodies from the complexes with ferritin that was caused by the existence of a cluster of overlapping immunodominant epitopes. After incubation for 1.5 h, the wells were washed, and the enzyme activity was determined with 0.02 M o-phenylenediamine and 0.02 M H2O2 in 0.1 M citrate-phosphate buffer (pH 5.0) for the substrate. The results were used to calculate the interaction constants in the system of double reciprocal values [5].
The ferritin-binding constants of the C5 and G10 antibodies were determined by competitive ELISA using ferritin immobilized in polystyrene plate wells and the biotin-labeled G10 antibodies. These antibodies effectively competed with the C5 antibodies for the epitopes on ferritin due to their overlapping [29]. The polystyrene plate wells were introduced with 50 ng of a conjugate of biotin with the G10 antibodies and increasing quantities of the antibodies, native or incubated with the organic solvents, in 0.2 ml of 0.05 M sodium phosphate buffer (pH 7.4) containing 1% BSA and 0.2 M NaCl. The wells were washed 1.5 h later and supplemented with a conjugate of streptavidin with horseradish peroxidase.
For graphic presentation, mean values from three independent determinations were used in all cases.
Spectra of the intrinsic fluorescence of the F11, G10, and C5 antibodies were recorded with an SFL-1211 spectrofluorometer (Solar, Minsk, Belarus) equipped with a personal computer using a cuvette with 1-cm optical path at 22 and 60°C. The protein samples (10-15 mg/ml) were diluted to the final concentration of 50 µg/ml with 0.1 M sodium borate buffer (pH 8.5) containing DMF (0-50%) and incubated for 15 min. The fluorescence spectra were recorded over the range 310-460 nm with excitation wavelength 295 nm.
To obtain denaturation curves, the stock solutions of the monoclonal
F11, G10, and C5 antibodies were diluted to the final concentration of
50 µg/ml with 0.1 M sodium borate buffer (pH 8.5) containing
increasing quantities of guanidine hydrochloride (0-5 M) or with the
buffer containing 10% DMF and the same quantities of guanidine
hydrochloride. The incubation was carried out overnight at room
temperature. Percent of the denatured fraction was determined by the
formula:
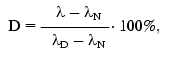
SDS-Electrophoresis in 15% polyacrylamide gel was carried out as described in [33] and staining with Coomassie R-250.
The antibody concentration was determined spectrophotometrically by the absorption at 280 nm [34] using the specific absorption coefficient A280 0.1% 1.62 that was calculated based on the known amino acid sequence according to [35].
RESULTS
The effect of DMF on the antigen binding by the monoclonal G10 and F11 antibodies (IgG2a subclass). Changes in the binding of ferritin by the G10 antibodies of higher affinity than the F11 antibodies [29] were analyzed by the method described in [31]. This method allowed us to determine parameters of the antibody binding with both the immobilized and soluble forms of the antigen and was used to exclude the dependence of the effect on both the analytical system design and the antigen form (soluble or immobilized ferritin).
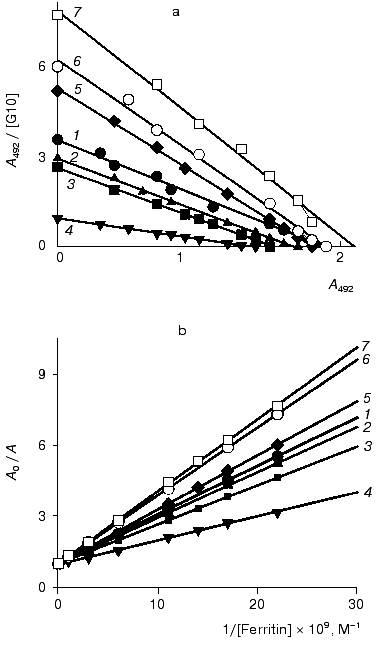
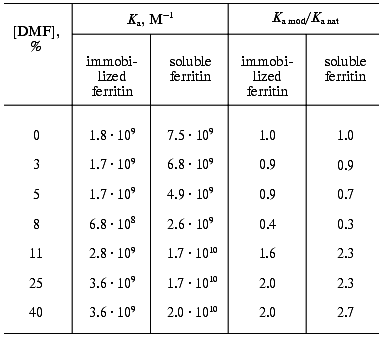
The interaction of the native and DMF-incubated G10 antibodies with ferritin immobilized by adsorption on polystyrene was determined by direct ELISA (Fig. 1a), and the interaction with the soluble ferritin was determined by competitive ELISA (Fig. 1b). Analysis of the binding constants (Table 1) shows that the incubation of the G10 antibodies with 11% DMF increased 1.6-fold the antibody affinity for the immobilized antigen, and the effect was retained at 25 and 40% DMF (Fig. 1a). In the case of the soluble antigen, the activation was 2.3-fold increased at 11% DMF, while the incubation with 40% DMF slightly increased the activation to 2.7-fold (Fig. 1b, Table 1).
Table 1. Ferritin-binding constants of the native and DMF-incubated G10 antibodies*Fig. 1. Determination of the ferritin-binding constants of the native and DMF-incubated monoclonal G10 antibodies by the method described in [31]. Concentrations of DMF: 0 (1), 3 (2), 5 (3), 8 (4), 11 (5), 25 (6), 40% (7). a) Determination of the interaction constants (Kax) of the native and DMF-incubated G10 antibodies with ferritin immobilized in polystyrene plate wells. The immune complexes were quantified with the anti-IgG-peroxidase conjugate. A492 is the peroxidase activity of the bound label, [G10] is the G10 antibody concentration. b) Determination of a relative parameter (r) to obtain the ferritin-binding constants (Kas) of the native and DMF-incubated G10 antibodies in solution. The immune complexes were developed with the anti-IgG-peroxidase conjugate. A0/A is the ratio of peroxidase activities in the absence (A0) and in the presence (A) of the soluble ferritin.
*The constants determined by the method of Hogg and Winzor [31].
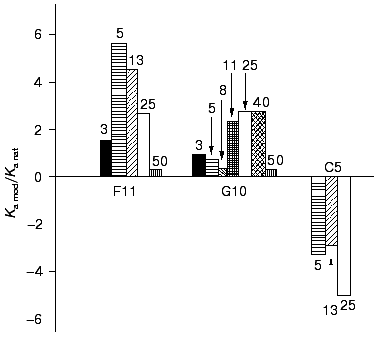
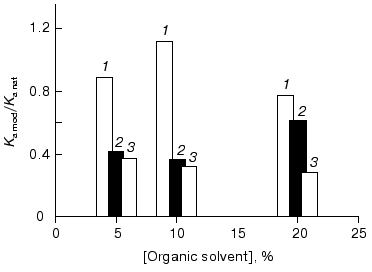
The effect of DMF on the G10 antibodies was characterized by a phase of decreased antigen-binding activity of the antibodies incubated in the presence of 3-8% DMF (Figs. 1 and 2). The antibody incubation with 8% DMF resulted in a threefold decrease in the binding constants of both the soluble and immobilized ferritin (Table 1). The binding constants of G10 incubated with 3-5% DMF with the immobilized antigen were not decreased, but the binding constants with the soluble antigen were decreased twofold. The G10 antibodies incubated with 50% DMF lost completely the ability to bind the antigen (Fig. 2).
The effects of the organic solvent on the binding activity of the antibodies did not depend on the method of determination of the constants, and this allowed us to use the competitive ELISA afterwards for calculation of the binding constants and for determination of effects of the organic solvent.Fig. 2. Effect of different concentrations of DMF on the antigen-binding ability of the monoclonal antibodies F11, G10, and C5. Ka mod/Ka nat is the ratio of the ferritin-binding constants of the native and DMF-incubated monoclonal antibodies. The incubation with ferritin was for 1 h. The binding constants were determined by competitive ELISA using the peroxidase conjugate of polyclonal anti-ferritin antibodies in the case of F11 antibodies and with biotin conjugate of the G10 antibodies for the G10 and C5 antibodies (see “Materials and Methods”) with the immobilized ferritin. The concentrations of ferritin are given in percent.
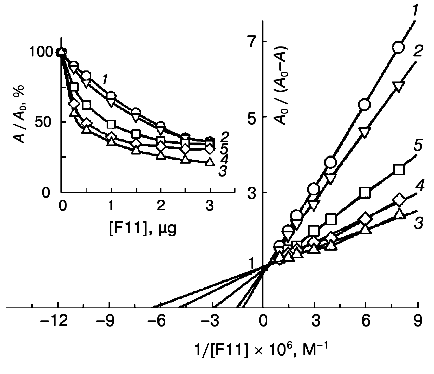
The monoclonal antibodies F11 (mouse IgG2a subclass) were also activated after incubation with DMF. These antibodies are directed against an epitope on the ferritin molecule spatially separated from the epitopes of the G10 and C5 antibodies and have a threefold lower affinity [29]. The binding of ferritin by the monoclonal F11 antibodies incubated with DMF (3-50%) was determined by competitive ELISA (Fig. 3). The antigen-binding constants of the antibodies were calculated from double reciprocal plots (Fig. 3), and they showed that the affinity of the antibodies after the incubation with DMF was 2.6-5.6-fold increased (Table 2), with the maximum activation at 5% DMF. Incubation in the presence of 3% DMF did not significantly influence the antigen-binding parameters of F11, whereas incubation in the presence of 50% DMF resulted in complete inactivation of the antibodies (Fig. 2).
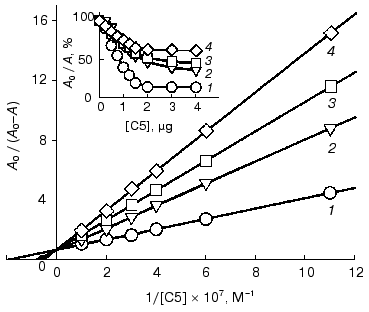
Table 2. Ferritin-binding constants of native and DMF-incubated F11 antibodies*Fig. 3. Determination of ferritin-binding constants of native and DMF-incubated monoclonal F11 antibodies by competitive ELISA from double reciprocal plots. Concentrations of DMF: 0 (1), 3 (2), 5 (3), 13 (4), 25% (5). A0/(A0 - A) is the ratio of the total activity of the peroxidase label (A0) to the activity of the free label in the solution (A0 - A). The insert presents the competitive ELISA of the native and DMF-incubated F11 antibodies with ferritin. A0/A (%) is the ratio of activities of the peroxidase label in the presence (A) and in the absence (A0) of the competitive agent.
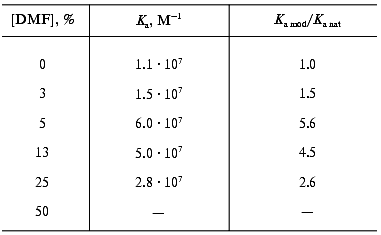
*The constants were determined from double reciprocal plots (here and further).
A sevenfold increase in antigen binding by the F11 antibodies was recorded earlier as a result of modification of one lysine residue per antigen molecule when DMF was used as the solvent of the modifier (palladium-coproporphyrin I) [5]. Comparison of the previous and present results suggests that the sevenfold increase in the antigen-binding parameters of the fluorescent porphyrin conjugates with the F11 antibodies can be attributed predominantly to the effect of DMF.
These findings suggest that conformational changes should occur in the antibodies after the incubation with the organic solvent DMF. These changes were irreversible and resulted in increased affinity of the monoclonal antibodies G10 and F11, and this increase depended on the concentration of the solvent. Activation of the F11 antibodies was observed even at 5% DMF, and in the case of the G10 antibodies the minimal activating concentration of DMF was 11%. But the activation of the F11 antibodies of low affinity was twofold higher than the activation of the high-affinity G11 antibodies of the same subclass (Tables 1 and 2). These differences are likely to be due to individual structural variations of the antibodies in the hypervariable regions of the antigen-binding sites.
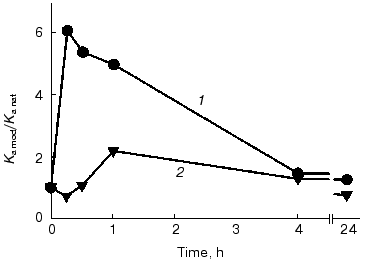
Time course of the DMF effect. The time of conformational changes induced by DMF in the antibodies and resulting in the functional activation was determined in the presence of maximal activating concentrations of the solvent, 5% for the F11 antibodies and 25% for the G10 antibodies. The effects were recorded as the binding constant determined by competitive ELISA (Fig. 4, Table 3). The maximal effect of 5% DMF on the F11 antibodies was observed within the first 15 min of the incubation, then the effect slightly decreased by the end of the first hour, with the subsequent decrease to the initial values after 24 h of the incubation (Fig. 4). Note that even the incubation of the F11 antibodies with DMF for more than 24 h did not cause a complete loss of the binding activity, which remained at the level corresponding to the activity of the native F11 (Fig. 4).
Table 3. Ferritin-binding constants of the F11 and G10 antibodies incubated with DMF for various timesFig. 4. Time course of ferritin binding by the F11 (1) and G10 (2) antibodies on incubation with DMF. Ka mod/Ka nat is the ratio of the binding constants of the native and DMF-incubated antibodies. The binding constants were determined by competitive ELISA using peroxidase conjugate with polyclonal anti-ferritin antibodies for the F11 antibodies and biotin conjugate with the G10 antibodies for the G10 antibodies (see “Materials and Methods”) with the immobilized ferritin.
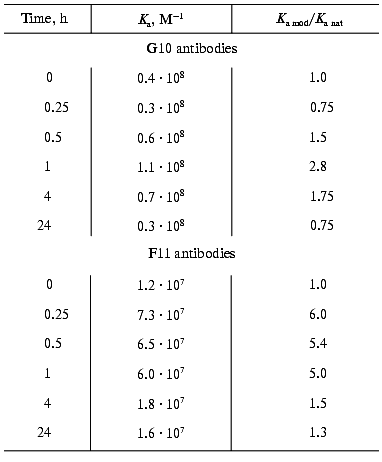
The maximal activation of the G10 antibodies was observed after 1 h of incubation with 25% DMF, and after 24 h of incubation the antigen binding was insignificantly decreased (Fig. 4, Table 3). These findings indicated that the exposure to the organic solvent DMF within 15-60 min was sufficient to induce conformational changes in the IgG2a molecule and that the kinetics of these conformational changes slightly varies in individual immunoglobulins of the same structural subclass. Insignificant changes in antigen-binding parameters of the antibody after incubation for 24 h indirectly indicate that the conformational changes were not global, because they did not result in significant changes in the long-term stability and activity of the antibodies.
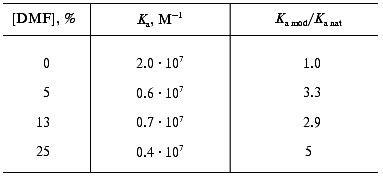
Effect of DMF on the antigen binding by the C5 antibodies of the IgG1 subclass. To study the effect of DMF on the binding activity of antibodies of the mouse IgG1 subclass, the monoclonal C5 antibodies were used. These antibodies are directed against an epitope on the ferritin molecule that is partially overlapped with the epitope of the G10 antibodies [29]. The C5 antibodies were incubated with 5, 13, 25% DMF, and the binding of ferritin was determined by competitive ELISA after the removal of the solvent. The increasing concentrations of DMF resulted in a monotonic 2.8-5-fold decrease in the ferritin-binding constants compared to the native antibodies (Figs. 2 and 5; Table 4). More than 3-fold decrease in affinity took place already at 5% DMF (Table 4). Such effect of DMF on the affinity of the C5 antibodies was likely to be due to specific structural features either of hypervariable regions of the antigen-binding domains or of labile structures in the constant region of the mouse IgG1 molecule.
Table 4. Ferritin-binding constants of the native and DMF-incubated C5 antibodiesFig. 5. Determination of ferritin-binding constants of native and DMF-incubated monoclonal C5 antibodies by competitive ELISA from double reciprocal plots. Concentrations of DMF: 0 (1), 5 (2), 13 (3), 25% (4). A0/(A0 - A) is the ratio of the total activity of the peroxidase label (A0) to the activity of the free label in the solution (A0 - A). The insert presents the competitive ELISA of native and DMF-incubated monoclonal C5 antibodies with ferritin. The immune complexes were developed using conjugate of streptavidin with peroxidase. A0/A (%) is the ratio of activities of the peroxidase label in the presence (A) and in the absence (A0) of the competitive agent.
Effects of other organic solvents on the functional activity of the G10 antibodies. In other experiments, organic solvents of various polarity were used, such as trifluoroethanol, dimethylsulfoxide, and methanol. The polarity of the organic solvents used decreases as follows: methanol > dimethylsulfoxide > trifluoroethanol [11]. The G10 antibodies (IgG2a subclass) were used as the model object because their thermodynamic stability is higher than that of the F11 antibodies [36, 37]. Effects of the organic solvents were compared in terms of their constants of antigen binding determined by competitive ELISA.
The G10 antibodies were incubated with methanol (5, 10, and 20%) for 1 h, and the ferritin-binding constants were found to decrease 1.7-3.5-fold (Fig. 6). The affinity was decreased after preincubation with 5% methanol.
Incubation of the G10 antibodies with dimethylsulfoxide (5, 10, and 20%) for 1 h also resulted in a monotonic decrease in the affinity of the antibodies: the ferritin-binding constants of the antibodies incubated with dimethylsulfoxide were decreased 2.7-3.6-fold (Fig. 6).Fig. 6. Effects of organic solvents (OS)--methanol (1), trifluoroethanol (2), and dimethylsulfoxide (3)--on the binding of ferritin by the monoclonal G10 antibodies. Ka mod/Ka nat is the ratio of the binding constants of the OS-incubated and the native G10 antibodies. The time of incubation with OS was 1 h. The binding constants were determined by competitive ELISA with immobilized ferritin using the biotin conjugate of the G10 antibodies (see “Materials and Methods”).
The incubation for 1 h with trifluoroethanol (5, 10, and 20%) virtually did not affect the affinity of the G10 antibodies: the binding constants were only 1.1-1.3-fold lower than those of the native antibodies (Fig. 6).
Thus, methanol, dimethylsulfoxide, and trifluoroethanol did not functionally activate the G10 antibodies; on the contrary, they seem to induce conformational changes resulting in a partial inactivation. Note that the inactivating effect decreased with the decrease in polarity of the organic solvents used. The incubation with trifluoroethanol, which is the least polar of the three solvents, had virtually no affect on the affinity of G10.
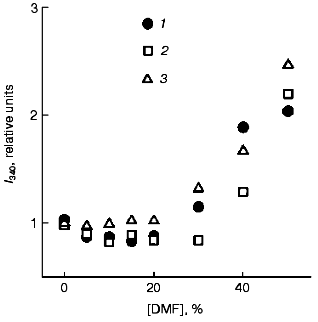
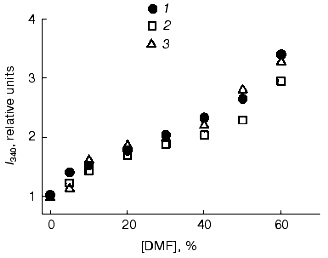
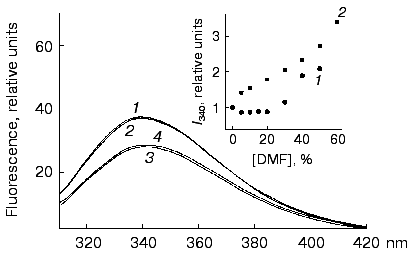
Fluorescent studies on the F11, G10, and C5 antibodies. No significant changes were found by analysis of the fluorescence maxima and intensities of the fluorophore surroundings in molecules of the F11, G10, and C5 antibodies in the presence of 0-30% DMF. Higher concentrations of DMF (up to 50%) increased the fluorescence 2-2.5-fold (Fig. 7) without changing the wavelength of the maximum fluorescence (338 nm). Thus, changes in the tertiary structure revealed by perturbations in the spatial surrounding of the aromatic fluorophores were not observed in the monoclonal F11, G10, and C5 antibodies at the concentrations of DMF (5-8%) sufficient for the development of the functional changes. To induce conformational changes in the presence of such low concentrations of DMF, an additional destabilizing force was required, e.g., an elevated temperature. At 60°C the fluorescence of the F11, G10, and C5 antibodies was 50-70% higher even in the presence of 10% DMF (Fig. 8). Further increase in DMF concentration increased nearly threefold the fluorescence of the three antibodies (at 50% DMF). Such an increase in the fluorescence has been suggested to be due to increase in the nonpolar microenvironment of the aromatic fluorophores due to an increased compactness of the protein and the imbedding of the fluorophores into the hydrophobic core of the molecule. This hypothesis seems to be unlikely because the imbedding of tryptophanyl residues into the hydrophobic nucleus would be accompanied by a wave shift in the maximal emission of the protein to shorter wavelength. Moreover, no effects of increased compactness of completely folded proteins are described for the case of functional native conformation, and they would contradict the concept that assumes the maximal compactness of native proteins. The increased fluorescence of the F11, G10, and C5 antibodies in the presence of DMF should be alternatively explained either by exposure of some of tryptophanyls to the less polar solvent, or by a spatial separation of aromatic fluorophores from intramolecular quenchers (presumably, disulfide groups) that are quenchers of the intrinsic fluorescence of native immunoglobulins [38-40]. In both cases, this suggests that the tertiary structure of the antibodies was partially disordered.
Fig. 7. Fluorescence intensities of the monoclonal antibodies F11 (1), G10 (2), and C5 (3) in the presence of DMF at 22°C. The excitation wavelength was 295 nm; the antibody concentration 50 µg/ml. The spectra were recorded after incubation of the samples with DMF for 15 min at 22°C.
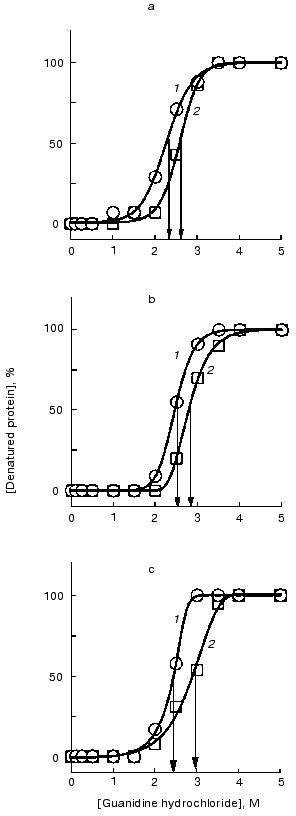
In the absence of pronounced changes in the tertiary structure of the F11, G10, and C5 antibodies at low concentrations of DMF (lower than 10%), we succeeded in recording a slightly decreased stability of the tertiary structure at 10% DMF when analyzing the denaturant-dependent unfolding of the native antibodies. This method was more sensitive to conformational changes because it permitted the description of not only the initial and final states of a protein but also the dynamics of a conformational transition. The denaturant concentration providing the half-maximal transition varied from 2 to 3 M for all three antibodies. For the native antibodies, we observed a small but reliably determined increase (0.3-0.5 M) in the same values (Fig. 9). These results suggest a small decrease in tertiary interactions responsible for stabilization of the F11, G10, and C5 antibody structure in the presence of 10% DMF.Fig. 8. Fluorescence intensities of the monoclonal antibodies F11 (1), G10 (2), and C5 (3) in the presence of DMF at 60°C. The excitation wavelength was 295 nm; the antibody concentration 50 µg/ml. The spectra were recorded after incubation of the samples with DMF for 15 min at 60°C.
Reversibility of changes in molecules of the F11 antibodies after incubation with DMF. Spectra of the intrinsic fluorescence of the F11 antibodies after incubation with 10% DMF and the removal of the organic solvent by dialysis did not differ from the spectra of the initial protein (Fig. 10). The same was found after the incubation of the F11 antibodies with 10% DMF at 60°C. Thus, conformational changes in the F11 antibodies induced by activating concentrations of DMF could not be revealed by the intrinsic fluorescence used to detect the global conformation of the protein. These results suggest that the conformational changes can be attributed to relatively small polypeptide segments that are not involved in the microenvironment of major fluorophores but essential for generation of the antigen-binding site of the antibody.Fig. 9. Denaturation curves of the monoclonal antibodies F11 (a), G10 (b), and C5 (c) in guanidine hydrochloride in the presence and in the absence of DMF: 1) the antibodies in the presence of 10% DMF; 2) the antibodies without DMF.
Fig. 10. Spectra of the intrinsic fluorescence of the monoclonal antibodies F11: 1, 3) the native antibodies; 2, 4) the antibodies incubated with 10% DMF. The spectra were recorded at 22 (1, 2) and 60°C (3, 4). DMF was removed by dialysis against sodium borate buffer (pH 8.5) overnight at 4°C. The antibody concentration and the time of incubation with 10% DMF was the same as in Fig. 7. The insert shows the fluorescence dependence of the monoclonal F11 antibodies on DMF concentration at 22 (1) and 60°C (2).
DISCUSSION
Incubation of two monoclonal antibodies of the mouse IgG2a subclass with DMF was found to functionally activate the antibodies, while methanol, dimethylsulfoxide, and trifluoroethanol had no activating effect. The degree of activation depended on the concentration of DMF and to a lesser extent on the antibody affinity. The low-affinity monoclonal antibodies F11 were activated twofold more (the maximal activation was 5.6-fold) than the high-affinity G10 antibodies. Moreover, to obtain the maximal activation of the low-affinity antibodies F11, a lower concentration of DMF was required (5 compared to 11% in the case of the G10 antibodies). The incubation with DMF of the C5 antibody that belongs to another antibody subclass (IgG1) but has affinity and specificity similar to the G10 antibodies [29] decreased the binding constant to the complete disappearance of the ability to interact with the antigen (Fig. 2).
Based on the functional analysis, the incubation of the monoclonal antibodies G10 and F11 of the mouse IgG2a subclass with DMF was concluded to cause irreversible conformational changes in the antibody molecules and the production of conformers that possess long-term stability after the organic solvent had been removed. These conformational changes occurred in the presence of relatively low concentrations of DMF (5-8%) that, as judged from the spectra of the intrinsic fluorescence (Fig. 7), could not induce global conformational changes in the structure of the immunoglobulins. These conformational changes presumably involved small and relatively disordered polypeptide segments of molecules that are relatively autonomous because of their weak interaction with the microenvironment. In immunoglobulins, two types of structure meet the mentioned requirements: hypervariable loops (CDR loops) of the antigen-binding regions in VL- and VH-domains, and the “hinge” region connecting the Fab- and Fc-fragments of antibodies [41]. The antibody conformers produced differed from the native molecules by the higher affinity for the antigen. Based on the fluorescence data (Figs. 8-10), it was suggested that the activated conformers possessed a slightly decreased local tertiary interactions, while their global tertiary structure remained unchanged.
Structural differences in the G10 and F11 antibodies of the same subclass are associated exclusively with the hypervariable loops. Together with certain differences in the effects of DMF, this is an indirect argument for the involvement of these structures in the molecular rearrangements and production of activated conformers induced by the organic solvent. However, the activating effect seems also to be subclass-specific, as we have observed the activation of the IgG2a antibodies but not of the C5 antibodies of the mouse IgG1 subclass. It is likely that specific structural features of the constant region of the IgG1 subclass restrict the organic solvent-induced conformational changes in the structural elements of the immunoglobulin. The differences between the immunoglobulin subclasses are known to be located mainly in the “hinge” region [41], and this is in good agreement with the findings of different rearrangements induced by DMF in the C5 antibodies and seems to indicate that the “hinge” region (at least, of the mouse IgG1 subclass) was involved in production of the antibody conformers.
The solvent specificity of the activation was an unexpected finding. Methanol, trifluoroethanol, and dimethylsulfoxide as inducers of conformational changes in proteins failed to functionally activate the G10 and F11 antibodies (Fig. 6). The incubation with these organic solvents resulted in production of a significant amount of insoluble antibody aggregations, and after the removal of soluble aggregations by gel filtration, the antigen-binding constants of the remaining antibodies in the solution were decreased. Thus, not only the concentration but also chemical features of the organic solvent were essential for conformational changes in the structures involved in the production of the activated conformers of the antibodies.
At present, limited knowledge on mechanisms of conformational changes in proteins and, especially, in immunoglobulins under the influence of low concentrations of organic solvents prevents to unequivocally explain some findings of our study. First, the reason for solvent specificity of the activation is unclear, as discussed above. Moreover, the identification of structures responsible for the subclass-dependent and individual (idiotypic) differences of the antibodies in the context of production of conformers with the increased or decreased affinity requires that numerous structural variants of antibodies be studied extensively. Finally, the functional effects induced by the organic solvent can also depend on the antigen, especially taking into account two large groups of antigens, proteins and low-molecular-weight substances (haptens). To elucidate these mechanisms and dependencies, further studies are required.
REFERENCES
1.Kirillova, N. M., Zhorov, O. V., Preygerson, V. A.,
and Martsev, S. P. (1991) Biokhimiya, 56, 1057-1068.
2.Ueno, H., Masuko, T., Wang, J., and Hashimoto, Y.
(1993) Biochem. Biophys. Res. Commun., 191, 701-708.
3.Martsev, S. P., Preygerson, V. A., Melnikova,Y. I.,
Kravchuk, Z. I., Ponomarev, G. V., Lunev,V. E., and Savitsky, A. P.
(1995) J. Immunol. Meth., 186, 295-304.
4.Bernard, M., Papini, E., Filippis, V., Gottardi,
E., Telford, J., Manetti, R., Fontanna, A., Pappuoli, R., and
Montecucco, C. (1995) J. Biol. Chem., 270,
23937-23940.
5.Melnikova, Y. I., Kravchuk, Z. I., Preygerson, V.
A., and Martsev, S. P. (1997) Biochemistry (Moscow), 62,
924-927.
6.Ritco-Vonsovici, M., Mouratou, B., Minard, Ph.,
Desmadril, M., Yon, J. M., Andrieux, M., Leroy, E., and Guttet, E.
(1995) Biochemistry, 34, 833-841.
7.Davies, D. R., and Cohen, G. H. (1996) Proc.
Natl. Acad. Sci. USA, 93, 7-12.
8.Franks, N. P., and Lieb, W. R. (1984)
Nature, 310, 599-601.
9.Timasheff, S. N., and Arakawa, T. (1988) in
Protein Function (Creighton, T. E., ed.) IRL Press, Oxford, New
York, Tokyo, pp. 331-348.
10.Narhi, L., Philo, J. S., Tiansheng, Li, Mei
Zhang, Samal, B., and Arakawa, T. (1996) Biochemistry,
35, 11447-11453.
11.Albert, J. S., and Hamilton, A. D. (1995)
Biochemistry, 34, 984-990.
12.Avdulov, N. A., Chochina, S. V., Daragan,V. A.,
Schroeder, F., Mayo, K. H., and Wood, W. G. (1996) Biochemistry,
35, 340-347.
13.Tams, J. W., and Welinder, K. G. (1996)
Biochemistry, 35, 7573-7579.
14.Ptitsyn, O. B. (1985) Proc. Int. Symp. Biomol.
Struct. Interactions, Suppl. J. Biosci., 8, 1-13.
15.Yoshino, T., Tsuchiya, T., and Kubota, Y. (1990)
Protein Eng., 3, 317.
16.Avbelj, F., and Moult, J. (1995)
Biochemistry, 34, 755-764.
17.Carra, J.-H., and Privalov, P. I. (1996) FASEB
J., 10, 67-73.
18.Shiraki, K., Nishikawa, K., and Goto, E. (1995)
J. Mol. Biol., 245, 180-194.
19.Briggs, M. S., and Roder, H. (1992) Proc.
Natl. Acad. Sci. USA, 89, 2017-2021.
20.Clore, G. M., Wingfield, P. T., and Gronenborn,
A. M. (1991) Biochemistry, 30, 2315-2323.
21.Varley, P., Gronenborn, A. V., Christenson, H.,
Wingfield, R. T., Pain, R. H., and Clore, G. M. (1993) Science,
260, 1110-1111.
22.Wei, J., and Fasman, G. D. (1995)
Biochemistry, 34, 6408-6415.
23.Lie, Z. P., Rizo, J., and Gierasch, L. (1994)
Biochemistry, 33, 134-142.
24.Yang, Y., and Mayo, K. H. (1993)
Biochemistry, 32, 8661-8671.
25.Bychkova, V. E., Dujsekina, A. E., Klenin, S. I.,
Tiktopulo, E. I., Uversky, V. N., and Ptitsyn, O. B. (1996)
Biochemistry, 35, 6058-6063.
26.Mimeault, M., de Lean, A., Lafleur, M.,
Bonenfant, D., and Fournier, A. (1995) Biochemistry, 34,
955-964.
27.Kotik, M., Radford, Sh. E., and Dobson, Ch. M.
(1995) Biochemistry, 34, 1714-1724.
28.Lunev, V. E., Melnikova, Y. I., Koshkin, S. A.,
Luneva, N. M., Cherkesova, T. S., Vasilevskaya, I. A., Preygerson, V.
A., and Martsev, S. P. (1993) Biochemistry (Moscow), 58,
491-501.
29.Melnikova, Y. I., Lunev, V. E., Preygerson, V.
A., Luneva, N. M., Koshkin, S. A., Rodionov, M. A., and Martsev, S. P.
(1993) Biochemistry (Moscow), 58, 502-511.
30.Zhorov, O. V., Kirillova, N. M., and Martsev, S.
P. (1991) Biokhimiya, 56, 828-838.
31.Hogg, F. J., and Winzor, D. J. (1987) Mol.
Immunol., 24, 797-801.
32.Reyes, M. A., Iriarte, A., and Martinez-Carrion,
M. (1993) J. Biol. Chem., 268, 22281-22291.
33.Laemmli, U. K. (1970) Nature, 227,
680-685.
34.Severin, S. E., and Solov'eva, G. A. (1989)
Manual in Biochemistry [in Russian], MGU Publishers, Moscow.
35.Gill, S. C., and Hippel, P. H. (1989) Analyt.
Biochem., 182, 319-326.
36.Chumanevich, A. A., Kravchuk, Z. I., Vlasov, A.
P., Zhorov, O. V., and Martsev, S. P. (1998) Biochemistry
(Moscow), 63, 476-484.
37.Kravchuk, Z. I., Chumanevich, A. A., Vlasov, A.
P., and Martsev, S. P. (1998) J. Immunol. Meth., 217,
131-141.
38.Tsunenaga, M., Goto, Y., Kawata, Y., and
Hamaguchi, K. (1987) Biochemistry, 26, 6044-6051.
39.Buchner, J., and Rudolph, R. (1991) Bio.
Technol., 9, 157-162.
40.Martsev, S., Kravchuk, Z., Chumanevich, A.,
Vlasov, A., Dubnovitsky, A., Bespalov, I., Arosio, A., and Deyev, S.
(1998) FEBS Lett., 441, 458-462.
41.Burton, D. R. (1985) Mol. Immunol.,
22, 161-206.