The Ca2+-Transport System of Yeast (Endomyces magnusii) Mitochondria: Independent Pathways for Ca2+ Uptake and Release
Y. I. Deryabina* and R. A. Zvyagilskaya
Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, Moscow, 117071 Russia; fax: (095) 954-2732; E-mail: inbio@online.ru* To whom correspondence should be addressed. Y. I. Deryabina was awarded the Prize for Young Scientists for the year 2000 by the Russian Biochemical Society.
Received July 6, 2000
Some features of the Ca2+-transport system in mitochondria of the yeast Endomyces magnusii are considered. The Ca2+ uniporter was shown to be specifically activated by low concentrations of physiological modulators such as ADP, NADH, spermine, and Ca2+ itself. The Na+-independent system responsible for Ca2+ release from Ca2+-preloaded yeast mitochondria was characterized. The rate of the Ca2+ release was proportional to the Ca2+ load, insensitive to cyclosporin A and to Na+, inhibited by La3+, TPP+, Pi, and nigericin, while being activated by spermine. We conclude that Ca2+ release from preloaded E. magnusii yeast mitochondria is mediated by a Na+-independent pathway, very similar to that in mitochondria from nonexcitable mammalian tissues. A scheme describing an arrangement of the Ca2+ transport system of yeast mitochondria is proposed.
KEY WORDS: yeast, Endomyces magnusii, mitochondria, calcium transport, regulation, ADP, NADH, calcium ions, Na+-independent calcium release
Abbreviations: RR) ruthenium red; CCCP) carbonyl cyanide m-chlorophenylhydrazone; TAN) translocase of adenine nucleotides; TPP+) tetraphenylphosphonium; CsA) cyclosporin A.
Ca2+ plays an important role in the control of basic
physiological functions by serving as an intracellular second messenger
[1, 2]. The
Ca2+-transport system is implicated in the signal
transduction pathway from the cytoplasm to the mitochondrial matrix to
stimulate respiration and oxidative phosphorylation [3, 4]. This transport system is
now receiving increasing attention as playing a central role in the
mechanism of cell apoptosis [5].
The calcium transport system of mammalian mitochondria is composed of two distinct components: 1) the Ca2+ uniporter, responsible for both energy-dependent Ca2+ uptake into the mitochondrial matrix [6, 7] and Ca2+ release caused by a drop in the transmembrane potential [8] or by a decrease in the Ca2+ concentration in incubation medium [9]; 2) specific pathways for Ca2+ release known as the Na+-dependent pathway (typical for mitochondria from excitable tissues) and the Na+-independent pathway (functional in mitochondria from nonexcitable animal tissues) [6, 8]. The Ca2+-uptake system is specifically inhibited by ruthenium red (RR) [10, 11] and lanthanides [10] and activated by various natural modulators including ADP [12], polyamines [13, 14], and Ca2+ itself [15]. The Na+-dependent pathway for Ca2+ efflux is inhibited by Mg2+, Mn2+, diltiazem, RR, and TPP+; the Na+-independent pathway is sensitive to Sr2+, Mn2+, and TPP+ [6].
Until recently, it was a general belief that yeast mitochondria, in contrast to mammalian mitochondria, lack an efficient Ca2+-transport system of any physiological relevance [16, 17]. However, in our laboratory we found that tightly coupled E. magnusii mitochondria have a highly efficient system for energy-dependent Ca2+ uptake by a uniport mechanism [18]. Later we showed that the kinetic properties of the Ca2+-transport system could be substantially changed in the presence of polyamines and Mg2+ [19, 20]. This study presents, in concise form, data on the regulation of the Ca2+-uptake system of yeast mitochondria by various natural modulators and on some features of the Ca2+-release system.
MATERIALS AND METHODS
BSA, EDTA, EGTA, sorbitol, mannitol, spermine, murexide, CaCl2, ADP, ATP, Tris, pyruvate, and malate were purchased from Sigma (USA); Coomassie G-250, safranine O, and NADH were from Serva (Germany); A23187 was from Boehringer Mannheim (Germany); dithiothreitol was from Reanal (Hungary). Other reagents of highest quality available were obtained from domestic suppliers.
The yeast E. magnusii, strain VKM Y-261, was grown as described earlier [21]. Mitochondria were isolated by a method developed in our laboratory and described previously [22]. Ca2+ uptake by mitochondria was assayed with a Hitachi-557 spectrophotometer using dual-wavelength photometry (507-540 nm) with murexide as a metallochromic indicator [23]. The incubation medium contained 0.6 M mannitol, 2 mM Tris-phosphate or 20 mM Tris-acetate, pH 7.4, 20 mM Tris-pyruvate, 5 mM Tris-malate, 50 µM murexide, and mitochondria corresponding to 0.5 mg protein/ml. Mitochondrial protein was assayed by the method of Bradford [24] with BSA as standard.
RESULTS AND DISCUSSION
Our first step was to search for natural modulators of the Ca2+-uptake system in yeast mitochondria. Low, close to physiological concentrations of ADP (15-25 µM) were found to specifically (other examined mono-, di-, and trinucleotides were ineffective) enhance Ca2+ accumulation by yeast mitochondria. The half-maximal effect was attained at 3 µM, which is comparable with the Km value for ADP binding to the translocase of adenine nucleotides (TAN) [12]. ADP is known as an inhibitor of the nonspecific permeabilization (pore) of the mammalian inner mitochondrial membrane; it acts by interacting with the TAN and locking it in the “M”-conformation, which is favorable for cation accumulation by mitochondria [12]. To test these two possibilities, we examined the rate of Ca2+ uptake as affected by atractyloside, a competitive inhibitor of the TAN, locking it in the “C”-conformation, and by cyclosporin A (CsA), a seemingly specific inhibitor of the Ca2+-dependent pore. Atractyloside totally prevented, while CsA had no impact on the stimulatory effect of ADP. We concluded therefore that the specific stimulatory effect of ADP was due to the locking of the TAN in the “M”-conformation. However, a direct effect of ADP on the Ca2+ uniporter could not be excluded [25].
Then we showed that NADH specifically stimulated Ca2+ uptake in yeast mitochondria. A peculiarity of the yeast respiratory chain is that it contains a dehydrogenase that oxidizes exogenous NADH. NADH, as we have demonstrated previously [26], is the main inducer of reverse electron transport, which determines the redox state of the mitochondrial pyridine nucleotides and, ultimately, the ability of mitochondria to accumulate and maintain large amounts of Ca2+. Yeast mitochondria after a short-term incubation with NADH displayed very high (record) rates of Ca2+ uptake and gained the capacity to retain the Ca2+ taken up. NADH may influence the transporter either directly or via modification of SH-groups of membrane proteins that play a crucial role in the ion-transport process.
Ca2+ itself was found to activate the Ca2+ uptake by yeast mitochondria. A short-term preincubation of the mitochondrial suspension with low Ca2+ concentrations accelerated the Ca2+ uptake and considerably improved the buffering properties of mitochondria due to an increased affinity of the system for Ca2+. It seems plausible that the Ca2+-induced stimulatory effect was due to allosteric activation of the uniporter as a result of interaction of the cation with regulatory sites of the uniporter [15].
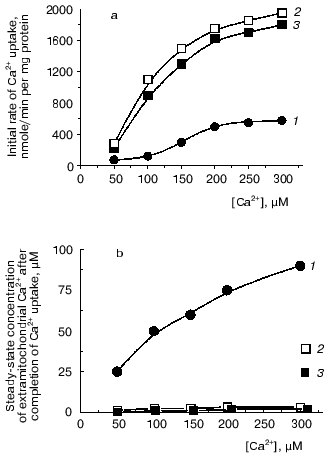
Added together, all physiological modulators examined, along with spermine, whose stimulatory effect on Ca2+ uptake by yeast mitochondria was shown by us earlier [20, 21, 25], provided significantly enhanced rates of Ca2+ uptake (Fig. 1a, 2) and the ability of yeast mitochondria to maintain a set-point at extramitochondrial Ca2+ concentrations comparable to the lower limit of sensitivity of the Ca2+ assay by the murexide technique (Fig. 1b, 2).
The stimulatory effect of modulators remained in the presence of concentrations of Mg2+, NaCl, and KCl mimicking the ionic composition of the cytoplasm (Fig. 1, 3). Thus, it was shown that E. magnusii mitochondria have a highly efficient system for Ca2+ uptake that is under the control of low concentrations of physiological modulators. This suggests its role in regulation of intramitochondrial Ca2+ and, ultimately, of mitochondrial oxidation, as was postulated for animal mitochondria [3, 4].Fig. 1. The combined effect of ADP, spermine, and preincubation of mitochondrial suspension with Ca2+ and NADH on the initial rate of Ca2+ uptake by yeast mitochondria (a) and steady-state concentration of Ca2+ in the incubation medium (b). The incubation medium contained 0.6 M mannitol, 2 mM Tris-phosphate, pH 7.4, 20 mM pyruvate, 5 mM malate, 50 µM murexide, and mitochondria corresponding to 0.5 mg protein/ml: 1) control; 2) in the presence of 25 µM ADP, 25 µM spermine and after a 1-min preincubation of mitochondrial suspension with 20 µM Ca2+ and 4 mM NADH; 3) the experimental conditions were as in (2) except that the incubation medium was supplemented with 0.5 mM MgSO4, 5 mM NaCl, and 100 mM KCl.
Indeed, we showed that NAD-dependent isocitrate dehydrogenase located in the mitochondrial matrix was activated by submicromolar Ca2+ concentrations, with a half-maximal effect at 100 nM Ca2+, which is comparable with the Ca2+ concentration in the matrix of animal mitochondria [6, 8].
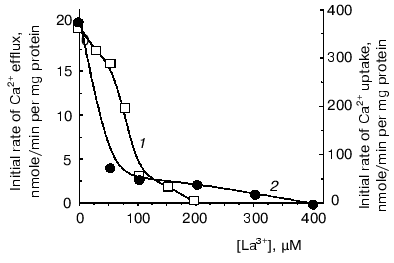
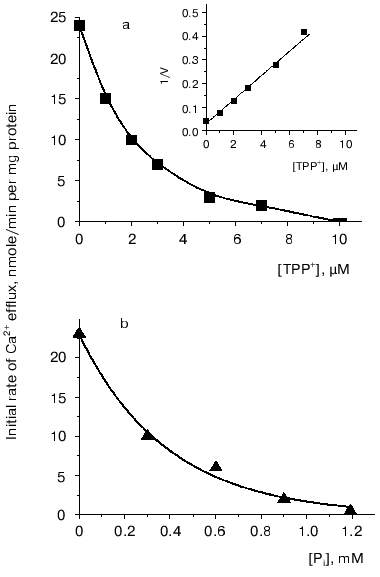
However, to postulate a role of a Ca2+-transport system in regulation of oxidative cellular metabolism, one should show the functioning of Ca2+-uptake-independent pathways for Ca2+ release from mitochondria. This was the goal of the second part of the study. Inorganic phosphate (Pi) is known as an inhibitor of Ca2+-uptake-independent pathways for Ca2+ efflux [27, 28]. Therefore, Ca2+ release from Ca2+-loaded mitochondria was examined using acetate in the incubation medium instead of phosphate as a permeant anion. In the acetate-containing medium, a spontaneous Ca2+ efflux ensued after uptake of about 75% of the added Ca2+. The rate of the Ca2+ release was proportional to the “Ca2+ load”. Spermine enhanced Ca2+ efflux. La3+, a competitive inhibitor of the Ca2+-uptake system, decreased the rate of both Ca2+ efflux (Fig. 2, 1) and Ca2+ uptake (Fig. 2, 2), albeit with distinct concentrations required for half-maximal and maximal inhibitory effects. This supports the view that reversed activity of the Ca2+ uniporter was not responsible for the Ca2+ release observed. CsA, an inhibitor of the Ca2+-dependent pore, did not affect Ca2+ release. This implies that the Ca2+ release from yeast mitochondria was not due to induction of the pore or reversed activity of the Ca2+ uniporter. In animal mitochondria, efflux of Ca2+ occurs via two specific Ca2+ release pathways, i.e., the Na+-dependent and the Na+-independent ones [6, 8]. Of these, the first could be definitely excluded in yeast mitochondria, as Ca2+ release was not stimulated by Na+. The Ca2+-efflux pathway in yeast mitochondria may thus be similar to the Na+-independent mechanism in animal mitochondria. This is supported by the fact that Ca2+ release was inhibited by TPP+ (Fig. 3a), a specific inhibitor of the Na+-independent pathway for Ca2+ release from animal mitochondria [8], with Ki of about 2 µM, which is close to the Ki value obtained for animal mitochondria.
Fig. 2. Effect of La3+ on initial rates of Ca2+ uptake (1) and Ca2+ efflux (2) in E. magnusii mitochondria. The incubation medium contained: 0.6 M mannitol, 20 mM Tris-acetate, pH 7.4, 20 mM pyruvate, 5 mM malate, 50 µM murexide, and mitochondria corresponding to 0.5 mg protein/ml.
Two main mechanisms are currently known for the Na+-independent Ca2+ release, i.e., a passive one driven by the DeltapH component of the membrane potential [28], and an active one using Deltaiota_as a driving force [29]. To obtain more information about the mechanism of the Ca2+ efflux from yeast mitochondria, we performed a series of experiments with the uncoupler CCCP, as well as with the K+ ionophores nigericin (decreasing DeltapH across the membrane) and valinomycin (decreasing Deltaiota across the membrane). The Ca2+ efflux was only insignificantly inhibited by CCCP, activated by valinomycin, and completely inhibited by nigericin. These results suggest that the Ca2+ release from the yeast mitochondria was primarily driven by the DeltapH. This suggestion was substantiated by the fact that the Ca2+ release was inhibited by low concentrations of Pi (Fig. 3b).Fig. 3. Effect of TPP+ (a) and Pi (b) on Ca2+ efflux from E. magnusii mitochondria. The experimental conditions were as described in the legend to Fig. 2.
The data presented demonstrate, to our knowledge for the first time, that mitochondria from a yeast species are endowed with not only an efficient Ca2+-uptake system which is under the control of low (physiological) concentrations of natural modulators, but also with a Ca2+-release system, most probably a Ca2+/2H+ exchanger, very similar to that in mitochondria from nonexcitable mammalian tissues [8].
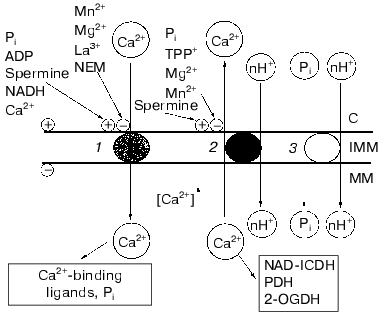
Figure 4 depicts a hypothetical scheme summarizing our results on the main principles of the arrangement of the Ca2+-transport system of yeast mitochondria. According to this scheme, the sequence of processes at the level of the inner mitochondrial membrane is the following: 1) an increase in the cytosolic Ca2+ would activate the Ca2+ uniporter, and this would establish a higher concentration of free Ca2+ in the mitochondrial matrix; 2) a rise of matrix Ca2+ concentrations would trigger Ca2+ efflux; 3) concerted operation of both the Ca2+-uptake and Ca2+-efflux systems would provide a certain steady-state concentration of Ca2+ in the matrix required to activate Ca2+-sensitive dehydrogenases of the Krebs cycle (notably, NAD-dependent isocitrate dehydrogenase), thus supplying more NADH and activating oxidative cellular metabolism.
This study was supported in part by the Russian Foundation for Basic Research (grants 97-04-49464 and 00-04-48277).Fig. 4. Scheme describing properties of the Ca2+ transport system in E. magnusii mitochondria and their modulation. Designations: 1) Ca2+ uniporter; 2) Ca2+/nH+ antiporter; 3) Pi/nH+ symporter; NAD-ICDH) NAD-dependent isocitrate dehydrogenase; PDH) pyruvate dehydrogenase complex; 2-OGDH) 2-oxoglutarate dehydrogenase complex; C) cytoplasm; IMM) inner mitochondrial membrane; MM) mitochondrial matrix; +) activation; -) inhibition.
REFERENCES
1.Clapham, D. E. (1995) Cell, 80,
259-268.
2.Mooren, F. Ch., and Kinne, R. K. H. (1998)
Biochim. Biophys. Acta, 1406, 127-151.
3.McCormack, J. G., and Denton, R. M. (1993)
Biochem. Soc. Trans., 21, 793-799.
4.Kavanagh, N. I., Ainscow, E. K., and Brand, M. D.
(2000) Biochim. Biophys. Acta, 1457, 57-70.
5.Bernardi, P., Scorrano, L., Colonna, R.,
Petronilli, V., and Di Lisa, F. (1999) Eur. J. Biochem.,
264, 687-701.
6.Gunter, T. E., Gunter, K. K., Sheu, S.-S., and
Gavin, C. E. (1994) Am. J. Physiol., 267, 313-339.
7.Zazueta, C., Zafra, G., Vera, G., Sanchez, C., and
Chavez, E. (1998) J. Bioenerg. Biomembr., 30,
489-498.
8.Gunter, T. E., and Pfeiffer, D. R. (1990) Am. J.
Physiol., 258, 755-786.
9.Igbavboa, U., and Pfeiffer, D. R. (1988) J.
Biol. Chem., 263, 1405-1412.
10.Reed, K. C., and Bygrave, F. L. (1974)
Biochem. J., 140, 143-115.
11.Broekemeier, K. M., Krebsbach, R. J., and
Pfeiffer, D. R. (1994) Mol. Cell Biochem., 139,
33-40.
12.Rottenberg, H., and Marbach, M. (1990)
Biochim. Biophys. Acta, 1016, 87-98.
13.Jensen, J. R., Lynch, G., and Baudry, M. (1987)
J. Neurochem., 48, 765-772.
14.Rustenbeck, J., Loptien, D., Fricke, K., Lenzen,
S., and Reiter, H. (1998) Biochem. Pharmacol., 56,
987-995.
15.Kriener, H. (1986) Arch. Biochem.
Biophys., 267, 525-535.
16.Carafoli, E., Balcavage, W. X., Lehninger, A. L.,
and Matton, J. R. (1970) Biochim. Biophys. Acta, 205,
18-26.
17.Balcavage, W. X., Lloyd, J. L., Matton, J. R.,
Ohnishi, T., and Scarpa, A. (1973) Biochim. Biophys. Acta,
305, 41-51.
18.Zvyagilskaya, R. A., Leikin, Y. N., Kozhokaru, N.
L., and Kotelnikova, A. V. (1983) Dokl. Akad. Nauk SSSR,
269, 1283-1290.
19.Votyakova, T. V., Bazhenova, E. N., and
Zvyagilskaya, R. A. (1990) FEBS Lett., 261, 139-141.
20.Votyakova, T. V., Bazhenova, E. N., and
Zvyagilskaya, R. A. (1993) J. Bioenerg. Biomembr., 25,
569-574.
21.Zvyagilskaya, R. A., Zelenshchikova, V. A.,
Uralskaya, L. A., and Kotelnikova, A. V. (1981) Biokhimiya,
46, 3-10.
22.Deryabina, Y. I., Bazhenova, E. N., and
Zvyagilskaya, R. A. (1996) Biochemistry (Moscow), 61,
1704-1713 (Russ.).
23.Scarpa, A. (1979) Meth. Enzymol.,
56, 301-338.
24.Bradford, M. M. (1976) Anal. Biochem.,
72, 248-254.
25.Bazhenova, E. N., Deryabina, Yu. I.,
Zvyagilskaya, R. A., Eriksson, O., and Saris, N.-E. (1998) J. Biol.
Chem., 273, 4372-4377.
26.Zvyagilskaya, R. A., Zelenshchikova, V. A., and
Burbaev, D. Sh. (1982) Dokl. Akad. Nauk SSSR, 263,
491-493.
27.Zoccarato, E., and Nicholls, D. (1982) Eur. J.
Biochem., 127, 333-338.
28.Rizzuto, R., Bernardi, P., Favoron, M., and
Azzone, G. F. (1987) Biochem. J., 246, 271-277.
29.Gunter, K. K., Zuscik, M. J., and Gunter, T. E.
(1991) J. Biol. Chem., 266, 21640-21648.