Two Exopolyphosphatases of the Cytosol of the Yeast S. cerevisiae: Comparative Characteristics
N. A. Andreeva, T. V. Kulakovskaya*, and I. S. Kulaev
Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Pushchino, Moscow Region, 142290 Russia; fax: (095) 923-3602; E-mail: alla@ibpm.serpukhov.su* To whom correspondence should be addressed.
Received September 28, 2000
In cell-free extracts of the yeast Saccharomyces cerevisiae that had been transferred from phosphate-deficient (-P) medium to complete (+P) medium (“hypercompensation” conditions), the specific and the total polyphosphatase activities increased (by 50 and 60%, respectively) compared to the control that was transferred from (+P) medium to (+P) medium. Specific and total polyphosphatase activities under “hypercompensation” conditions increased by 25 and 43% in cytosol, by 33 and 100% in vacuoles, and by 50 and 50% in the total membrane fraction, respectively. In contrast, the polyphosphatase activity in the cell envelope somewhat decreased compared to the control. Under the growth conditions indicated above, a novel high molecular weight polyphosphatase was revealed in the cytosol fraction along with the previously studied 40-kD polyphosphatase. Unlike the 40-kD polyphosphatase, which is most active with tripolyphosphate, this novel enzyme has a molecular mass of more than 440 kD and is most active with high molecular weight polyphosphates. This polyphosphatase is insensitive to antibodies that suppress the activity of the 40-kD polyphosphatase of the cytosol. In a number of properties, the high molecular weight polyphosphatase of the cytosol resembles the polyphosphatase of vacuoles, but it differs from the polyphosphatases of nuclei and mitochondria of S. cerevisiae. The ratio of the low and high molecular weight polyphosphatases depends on the culture growth conditions. Under “hypercompensation” conditions, the total activity of the high molecular weight polyphosphatase in the cytosol is five times higher than that of the 40-kD polyphosphatase. During growth without re-inoculation, the 40-kD polyphosphatase is predominant in the cytosol; its total activity in dependence on the growth stage is 3.5-12.5 times higher than the activity of the high molecular weight form.
KEY WORDS: polyphosphates, exopolyphosphatase, cytosol, hypercompensation, yeast, Saccharomyces cerevisiae
Abbreviation: polyPn) inorganic polyphosphates, where n is the average number of phosphate residues in the chain.
Inorganic polyphosphates (polyP) play an important role in yeasts for
withstanding unfavorable conditions such as Pi deficiency
and the presence of heavy metal cations in the medium [1, 2]. For example, in the absence
of Pi in the medium, Saccharomyces cerevisiae yeast
cells are able to essentially completely use its stores in the form of
polyP to maintain their vital activity [2].
Re-inoculation of yeast cells from Pi-deficient to complete
medium results in enhanced accumulation of polyP compared to the
control [1, 2]. This phenomenon
is called “hypercompensation”. The mechanisms allowing yeast
cells to regulate the polyP concentration in various compartments are
still unstudied. Exopolyphosphatase (polyphosphate phosphohydrolase, EC
3.6.1.11, hereafter called polyphosphatase) is one of the enzymes that
may participate in these processes. The several exopolyphosphatases in
S. cerevisiae differ in location within the cells and in
physicochemical properties [3]. It is not known how
the growth conditions influence the concentration and activity of
various polyphosphatases in yeast cells. Elucidation of this influence
may help to determine the functions of yeast polyphosphatases and the
mechanisms regulating their activity.
As will be demonstrated in this work, under conditions when S. cerevisiae cells actively synthesize polyP a novel high molecular weight polyphosphatase appears in their cytosol; the properties of this novel polyphosphatase differ from those of the 40-kD polyphosphatase of this compartment studied earlier [4, 5].
MATERIALS AND METHODS
Saccharomyces cerevisiae BKM Y-1173 (IBPM-366) was grown on a shaker in flasks with 200 ml of Reader medium at 30°C [6].
Two growth procedures were used. In the standard procedure, biomass grown on complete Reader medium (+P) with 18.3 mM Pi was taken for analysis at points on the growth curve corresponding to early logarithmic (A600 = 1), mid-logarithmic (A600 = 3), and stationary (A600 = 7.2) phases.
In the procedure with re-inoculation, the culture was grown to the late logarithmic phase (A600 = 5) on Reader medium (+P) or on Reader medium (-P). Potassium phosphates were not added to (-P) medium, but a small amount of phosphate (1.3 mM) was added with yeast extract. Biomass was collected by centrifugation at 5,000g for 10 min, washed with distilled water, and placed on fresh medium (+P) so that the initial density of the culture remained the same (A600 = 5). After cultivation for 5 or 16 h, the cells were centrifuged, washed with distilled water as above, and used for further analysis.
To obtain cell-free extract, the biomass was disintegrated by extrusion in the frozen state using an IBPM press, then suspended in 50 mM Tris-HCl buffer (pH 7.2) containing 0.5 mM phenylmethylsulfonyl fluoride and centrifuged at 100,000g for 60 min.
To obtain vacuoles, cytosol and the residual membrane fraction from one sample of biomass, protoplasts obtained as described earlier [7], were disintegrated in 20 mM Tris-HCl buffer (pH 7.2) containing 0.1 M sorbitol and 6% Ficoll. Vacuoles were obtained by flotation in a stepwise gradient of concentration to a layer containing 4% Ficoll [7]. The remainder of the membrane structures thus appeared in the pellet, which was analyzed. The supernatant in a layer containing 6% Ficoll was taken as the cytosol fraction. To determine enzymatic activities, vacuoles were disintegrated [7]. Polyphosphatase of the cell envelope was extracted from whole cells [8]. For gel filtration, cytosol was obtained by disintegration of protoplasts and precipitation of the total organelle fraction [5].
Polyphosphatase and pyrophosphatase activities were determined in 1 ml of the reaction mixture for 10-30 min at 30°C via the rate of formation of Pi. The reaction was initiated by addition of the enzyme. Pi formed during the reaction was determined with ascorbic acid and SDS [9]. The incubation medium for determination of polyphosphatase and pyrophosphatase activities contained 50 mM Tris-HCl buffer (pH 7.2), 2.5 mM MgSO4 and 0.005 mM polyP208 or 1 mM PPi. Cases when other substrates or other incubation media were used are specified individually. To determine the pH optimum for activity, 100 mM Tris-acetate buffer, pH 5-9, was used. To determine the effect of reagents, the sample was preincubated with them in the above-mentioned incubation mixture for 5 min at 20°C. The reaction was initiated by addition of the substrate. To determine the effect of cations, samples after dialysis against 25 mM Tris-HCl buffer (pH 7.2) containing 0.1% Triton X-100 for 4 h were used. Nonspecific phosphatase activity was determined as described earlier [8].
One activity unit (U) was defined as the enzyme quantity sufficient to catalyze the formation of 1 µmole Pi in 1 min.
PolyP with average chain length of 9, 15, and 208 orthophosphate residues (Monsanto, USA) were freed from Pi and PPi by gel filtration on Sephadex G-10 [8]. The average chain length of polyP was taken as that specified by the producer. PolyP3 (Sigma, USA) and PPi (Koch-Light, Great Britain) were used without purification.
Protein concentrations were determined using bovine serum albumin as the standard [10]. Polyclonal rabbit antiserum to the purified polyphosphatase of the S. cerevisiae yeast cell envelope [11] was used at dilution 1 : 500.
Gel filtration of cytosol preparations was performed on a 1.6 × 80-cm Sephacryl S-300 column (Pharmacia, Sweden) equilibrated with 20 mM Tris-HCl buffer (pH 7.2) containing 2 mM MgSO4, 100 mM NaCl, and 0.1% Triton X-100. Ribonuclease (13.7 kD), chymotrypsinogen A (25 kD), ovalbumin (43 kD), bovine serum albumin (67 kD), aldolase (158 kD), catalase (232 kD), and ferritin (440 kD) (Pharmacia, Sweden, and Serva, Germany) were used as the standards.
RESULTS AND DISCUSSION
An increase in the polyP concentration in cells is observed on re-inoculation of S. cerevisiae from phosphate-deficient to complete medium [1, 2].
We found that on re-inoculation of S. cerevisiae from (-P) to (+P) medium both specific and total polyphosphatase activities of cell-free extract are higher than for the control that was re-inoculated from (+P) to (+P) medium. The difference is larger after cultivation for 16 h than for 5 h (Table 1). Thus, for further experiments cultivation for 16 h after re-inoculation was used. The amounts of biomass were similar in both procedures (Table 2).
Table 1. Polyphosphatase activity of a
cell-free extract of S. cerevisiae on re-inoculation of cells
grown to late logarithmic phase to fresh medium
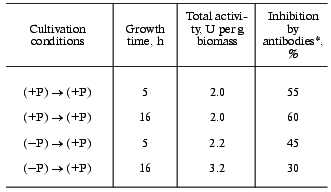
*Antibodies inhibiting the 40-kD polyphosphatase of
cytosol.
Table 2. Effect of various growth procedures
on the growth and polyphosphatase activity of the S. cerevisiae
cytosol fraction
The sensitivity of polyphosphatase activity of a cell-free extract to antibodies against the purified polyphosphatase of the cell envelope is significantly lower on re-inoculation from (-P) to (+P) medium (Table 1). These antibodies by 70-90% suppress the activity of polyphosphatases of the cell envelope and cytosol purified by us earlier [4, 11] and do not inhibit polyphosphatases of vacuoles, nuclei, and mitochondria of the same yeast [3, 12, 13]. Consequently, under “hypercompensation” conditions, the contribution to the total cell activity from polyphosphatases insensitive to these antibodies increases. The pyrophosphatase activity was equal for both procedures (data not presented here).
To determine the contributions of polyphosphatases of various compartments to the total activity increase under “hypercompensation” conditions, the cytosol, vacuole, and the total membrane fractions from the same protoplast sample were isolated. The polyphosphatase of the cell envelope was extracted from whole cells grown under the same conditions.
Under “hypercompensation” conditions, the total polyphosphatase activity in cytosol is 1.4 times higher than that on re-inoculation from (+P) to (+P) medium. The specific activity is also higher (Table 3). In vacuoles on re-inoculation from (-P) to (+P) medium, the specific and total polyphosphatase activities are 1.3 and 2 times higher, respectively, than those on re-inoculation from (+P) to (+P) medium (Table 3). The total membrane fraction remaining after isolation of vacuoles is also characterized by higher specific and total polyphosphatase activities compared to the control (Table 3). In contrast, the specific and total polyphosphatase activities in the cell envelope decreased somewhat under “hypercompensation” conditions (Table 3). However, the specific and total activities in the extract from this compartment were 3-4 times lower for both growth procedures than with the standard yeast growth to mid-logarithmic phase [8].
Table 3. Polyphosphatase activities of
various cell compartments of S. cerevisiae on re-inoculation of
cells grown to late logarithmic phase to fresh medium
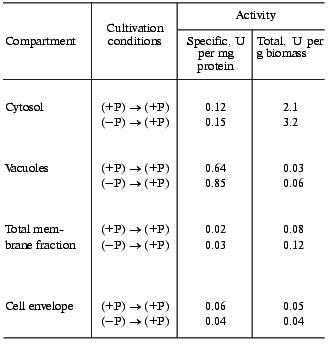
Thus, during “hypercompensation” the specific and total polyphosphatase activities in the cytosol, vacuole, and total membrane fractions are higher than those during growth under the control conditions. But the cytosol fraction is the main contributor to the increase in the total polyphosphatase activity of the cell, so further experiments were performed with this fraction.
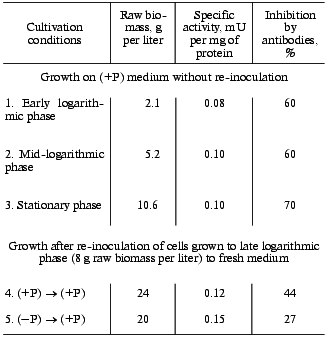
We compared the growth and specific polyphosphatase activity and its sensitivity to antibodies for the cytosol fraction under the standard growth procedure and under growth with re-inoculation. On growth with re-inoculation to fresh medium, both from (-P) to (+P) and from (+P) to (+P), biomass increased approximately twice compared to the stationary phase of the standard growth procedure (Table 2). The specific polyphosphatase activity of the cytosol fraction and its sensitivity to antibodies changed only slightly in various growth phases under the standard growth procedure (Table 2). Compared to the standard growth, on re-inoculation from (+P) to (+P) some increase in the specific polyphosphatase activity of cytosol and decrease in its sensitivity to antibodies is observed. These changes are even more obvious on re-inoculation from (-P) to (+P).
Since the sensitivity of cytosol polyphosphatase activity to antibodies significantly decreased on re-inoculation of the cells grown to the late logarithmic phase to fresh medium, especially under “hypercompensation” conditions, it seems that under these conditions the sensitivity of the well-known 40-kD polyphosphatase to antibodies changes, or another polyphosphatase appears in the cytosol.
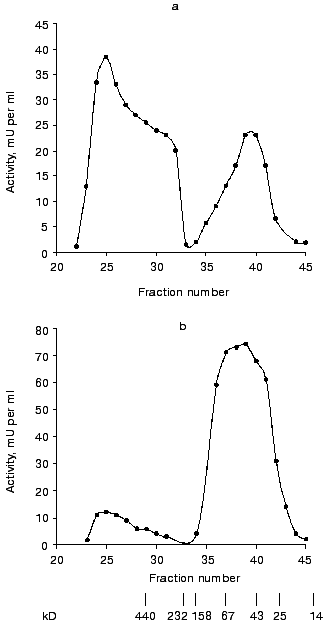
To differentiate between these possibilities, cytosol preparations were subjected to gel filtration. The elution profile of a cytosol preparation obtained from cells grown according to the (-P) to (+P) scheme is presented in Fig. 1a. An additional peak with molecular mass of more than 440 kD was observed along with the peak of the earlier purified cytosolic polyphosphatase with molecular mass of approximately 40 kD [5]. Both the specific and total activities are higher in the total fraction of high molecular weight polyphosphatase than in the total fraction of 40-kD polyphosphatase (Table 4). The contribution of the high molecular weight polyphosphatase to the total polyphosphatase activity of the cytosol preparation was 84%. A high molecular weight protein peak was also observed in the cytosol preparation of cells grown after re-inoculation from (+P) to (+P) medium. However, both specific and total activities of the total fraction of the high molecular weight polyphosphatase were lower (Table 4).
Table 4. Polyphosphatase activity of fractions of various molecular masses obtained by gel filtration of cytosol preparations on Sephacryl S-300Fig. 1. Polyphosphatase activity in Sephacryl S-300 gel-filtration fractions of S. cerevisiae cytosol preparations. Positions of protein markers with molecular masses 440, 232, 158, 67, 43, 25, and 14 kD are shown by the vertical lines. a) Cytosol preparation was obtained from cells grown with re-inoculation from (-P) to (+P) medium; 36 mg of protein and 3.8 U of polyphosphatase activity were applied onto the column. b) Cytosol preparation was obtained from cells grown to mid-logarithmic phase on (+P) medium without re-inoculation; 31 mg of protein and 3.2 U of polyphosphatase activity were applied onto the column.
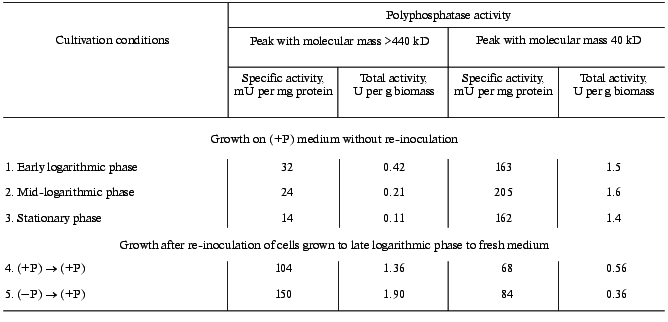
Gel filtration of cytosol preparations obtained from cells in various growth phases with the standard growth procedure was performed as a control experiment. The elution profile of the cytosol preparation from yeast cells grown to mid-logarithmic phase is presented in Fig. 1b. As compared to Fig. 1a, there are significantly smaller amounts of the high molecular weight polyphosphatase and larger amounts of the 40-kD polyphosphatase. The contribution of the high molecular weight polyphosphatase to the total activity of the cytosol preparation during growth on Reader medium decreased from 22% in the early logarithmic phase to 12% in the mid-logarithmic phase and to 7% in the stationary phase. In all the growth phases the specific activities of the total fraction of high molecular weight polyphosphatase were significantly lower than those of the low molecular weight enzyme (Table 4).
Thus, the 40-kD enzyme is the main polyphosphatase of S. cerevisiae cytosol in all the growth phases with the standard growth procedure on Reader medium. On re-inoculation of cells grown to the late logarithmic phase on fresh medium, the high molecular weight polyphosphatase predominates in the cytosol. Although this phenomenon is most obvious on “hypercompensation”, it is also observed on transfer from (+P) to (+P) medium. We suggest that the appearance of the high molecular weight polyphosphatase in S. cerevisiae cytosol is somehow related an increase in the synthesis of polyP. The rate of polyP accumulation in microorganisms depends not only on the phosphorus concentration in the medium, but also on energy-releasing processes including glycolysis [1]. That is why transfer to fresh medium can result in enhancement of synthesis of polyP not only due to “hypercompensation”, but also due to enhancement of glycolysis. There is a lack of glucose in the medium at the end of logarithmic phase when re-inoculation is performed [2]. The maximal activity of the high molecular weight polyphosphatase in cytosol is observed in the early logarithmic phase during the standard growth procedure, but on further cultivation its activity gradually decreases (Table 4).
Using the pooled fractions of the high molecular weight polyphosphatase peak, we determined some properties of the enzyme.
The purified 40-kD polyphosphatase is most active with polyP3; its activity with this substrate is 1.5 times higher than with polyP208 [4]. In contrast, the high molecular weight polyphosphatase of the cytosol more actively hydrolyses high molecular weight polyP (Table 5), and its activity with polyP3 is only 13% of that with polyP208. Moreover, polyP3 hydrolysis can be partly caused by the pyrophosphatase activity present in this preparation (data not presented here). However, the latter is insensitive to heparin and is essentially completely suppressed by 1 mM fluoride. Pyrophosphatase seems to be present in this preparation. Nonspecific phosphatase activity is practically absent in the studied fraction (Table 5).
Table 5. Substrate specificity of two
polyphosphatases of S. cerevisiae cytosol
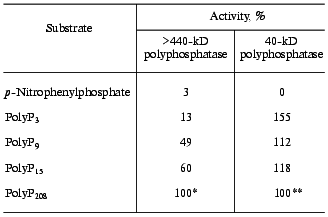
*100% corresponds to activity of 0.17 U per mg protein. A
preparation after gel filtration on Sephacryl S-300 was used.
**100% corresponds to activity of 270 U per mg protein. Data
for the purified enzyme were taken from [4].
The effect of some reagents on the activity of the pooled high molecular weight polyphosphatase fractions after gel filtration is shown in Table 6. Similar to polyphosphatases of vacuoles, nuclei, and mitochondria [3, 11-13], it was completely insensitive to the antibodies against the purified phosphatase of the cell envelope. The polyphosphatase activity of the 40-kD peak was inhibited by these antibodies to the same extent (70%) as the earlier purified cytosolic polyphosphatase [4]. EDTA, which stimulates the 40-kD polyphosphatase of the cytosol [4], inhibited the high molecular weight polyphosphatase (Table 6). The sensitivity of the high molecular weight polyphosphatase to fluoride is another distinction between these two enzymes (Table 6). Heparin, which inhibits yeast polyphosphatases of all compartments [2], also suppressed this activity.
Table 6. Effect of some reagents on activity
of the two polyphosphatases of S. cerevisiae cytosol
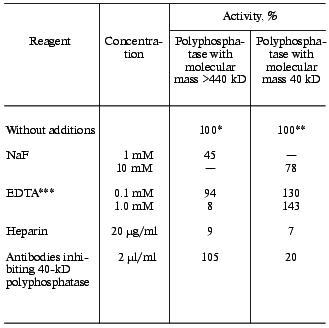
*100% corresponds to activity of 0.17 U per mg protein with
polyP208. The pooled fractions of the peak after gel
filtration on Sephacryl S-300 was used.
**100% corresponds to activity of 320 U per mg protein with
polyP15. Data for the purified enzyme were taken from [4].
***Mg2+ concentration, 1 mM.
The pH optimum for the activity of the high molecular weight polyphosphatase was close to 7, like that for other yeast polyphosphatases [2]. However, the pH optimum was sharper than that of the 40-kD polyphosphatase [14]. Thus, at pH 5.0 the activity was 19% and at pH 9.0 only 10% of the maximal activity at pH 7.2.
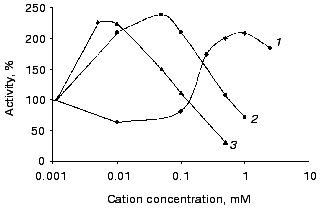
Similar to most other polyphosphatases, the studied activity is stimulated by bivalent cations (Fig. 2). However, the level of this stimulation is not high and is essentially the same for Zn2+, Co2+, and Mg2+. The ammonium cation has the largest stimulatory effect: it increased activity by 50-70% both in the absence and in the presence of a bivalent cation.
So, the high molecular weight polyphosphatase of cytosol markedly differs in its properties from the 40-kD polyphosphatase of this compartment. On transfer of S. cerevisiae cells grown to the late logarithmic phase to fresh medium, its activity in cytosol increased 7-9-fold. However, the activity of the 40-kD polyphosphatase decreased 2.5-4-fold (Table 4); the latter is the main polyphosphatase of cytosol on growth without additional re-inoculation.Fig. 2. Effect of Mg2+ (1), Co2+ (2), and Zn2+ (3) on the activity of the high molecular weight polyphosphatase of cytosol with polyP208. The pooled fractions with molecular mass >440 kD after gel filtration was used.
In some properties the high molecular weight polyphosphatase of cytosol is similar to the polyphosphatase isolated from vacuoles [15]. Both of them have high molecular masses, are more active with high molecular weight polyP, are not inhibited by antibodies against polyphosphatase of the cell envelope, are not inhibited by EDTA, and the levels of their stimulation by bivalent cations are similar. The other polyphosphatases of other compartments of the same yeast do not exhibit such similarity to the high molecular weight polyphosphatase of cytosol [2].
Perhaps the high molecular mass of the new polyphosphatase detected in cytosol is due to its forming a complex with other proteins and/or polyP. The pooled fractions of its peak after gel filtration contains labile phosphorus at concentration about 365 µM. It is known that polyP can be contained in yeast cell cytosol as volutin granules [1]. These structures isolated from yeast cells contain four proteins with unknown function along with polyP [16].
It was shown that on “hypercompensation”, during the first hours low molecular weight polyphosphates are synthesized, and then a drastic growth of the chain length of these compounds occurs [17]. The appearance of the high molecular weight polyphosphatase in cytosol, which can hydrolyze long-chain polyP more efficiently, is possibly related with a need to regulate their quantity. Changes in the activities of the individual forms of enzymes during growth and in dependence on cultivation conditions are typical of various microorganisms. In particular, the mechanism of this phenomenon is well studied for beta-glucosidases [18]. To clarify the origin and functions of the high molecular weight polyphosphatase of cytosol, further investigations are needed.
This work was supported by the Russian Foundation for Basic Research (grants No. 99-04-48246 and 00-15-97851) and by INCO Copernicus (PL 971185).
REFERENCES
1.Kulaev, I. S. (1975) Biochemistry of
High-Molecular-Weight Polyphosphates [in Russian], MGU Publishers,
Moscow.
2.Kulaev, I. S., Vagabov, V. M., and Kulakovskaya, T.
V. (1999) J. Biosc. Bioeng., 88, 111-129.
3.Kulaev, I. S., Vagabov, V. M., Kulakovskaya, T. V.,
Lichko, L. P., Andreeva, N. A., and Trilisenko, L. B. (2000)
Biochemistry (Moscow), 65, 271-278.
4.Andreeva, N. A., Kulakovskaya, T. V., and Kulaev,
I. S. (1996) Biochemistry (Moscow), 61, 1213-1220.
5.Andreeva, N., Kulakovskaya, T., Sidorov, I.,
Karpov, A., and Kulaev, I. (1998) Yeast, 14, 383-390.
6.Okorokov, L. A., Lichko, L. P., and Kulaev, I. S.
(1980) J. Bacteriol., 144, 661-665.
7.Andreeva, N. A., Lichko, L. P., Kulakovskaya, T.
V., and Okorokov, L. A. (1993) Biochemistry (Moscow), 58,
737-744.
8.Andreeva, N. A., and Okorokov, L. A. (1993)
Yeast, 9, 127-139.
9.Kulakovskaya, T. V., Andreeva, N. A., Karpov, A.
V., Sidorov, I. A., and Kulaev, I. S. (1999) Biochemistry
(Moscow), 64, 990-993.
10.Bensadoun, A., and Weinstein, D. (1976)
Analyt. Biochem., 270, 241-250.
11.Kulakovskaya, T. V., Andreeva, N. A., Lichko, L.
P., and Kulaev, I. S. (1995) Biochemistry (Moscow), 60,
1569-1572.
12.Lichko, L. P., Kulakovskaya, T. V., and Kulaev,
I. S. (1996) Biochemistry (Moscow), 61, 361-366.
13.Lichko, L. P., Kulakovskaya, T. V., and Kulaev,
I. S. (1998) Biochim. Biophys. Acta, 1372, 153-162.
14.Andreeva, N. A., Kulakovskaya, T. V., and Kulaev,
I. S. (1994) Biochemistry (Moscow), 59, 1411-1418.
15.Andreeva, N. A., Kulakovskaya, T. V., and Kulaev,
I. S. (1998) FEBS Lett., 429, 194-196.
16.Jacobson, L., Helman, M., and Yariv, J. (1982)
Biochem. J., 201, 437-439.
17.Vagabov, V. M., Trilisenko, L. V., and Kulaev, I.
S. (2000) Biochemistry (Moscow), 65, 349-354.
18.Staykova, D., Ivanova, G. S., and Kulaev, I. S.
(1984) Biokhimiya, 49, 1583-1587.