Comparative Study of Effects of Artificial Electron Donors on the AT-Band of Photosystem II Thermoluminescence
M. Yu. Kultisheva1*, E. R. Lovyagina1, A. M. Kuznetsov2, M. K. Solntsev2, B. K. Semin1, and I. I. Ivanov1
1School of Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-1115; E-mail: kulticheva@mars.biophys.msu.ru; loviagina@mars.biophys.msu.ru; semin@mars.biophys.msu.ru; ivanov@mars.biophys.msu.ru2School of Physics, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-1195; E-mail: mks@bio471c.phys.msu.ru
* To whom correspondence should be addressed.
Received December 22, 2000; Revision received March 2, 2001
Extraction of the Mn-cluster from photosystem II (PS II) inhibits the main bands of thermoluminescence and induces a new AT-band at -20°C. This band is attributed to the charge recombination between acceptor QA- and a redox-active histidine residue on the donor side of PS II. The effect of Mn(II) and Fe(II) cations as well as the artificial donors diphenylcarbazide and hydroxylamine on the AT-band of thermoluminescence was studied to elucidate the role of the redox-active His residue in binding to the Mn(II) and Fe(II). At the Mn/PS II reaction center (RC) ratio of 90 : 1 and Fe/PS II RC ratio of 120 : 1, treatment with Mn(II) and Fe(II) causes only 60% inhibition of the AT-band. Preliminary exposure of Mn-depleted PS II preparations to light in the presence of Mn(II) and Fe(II) causes binding of the cations to the high-affinity Mn-binding site, thereby inhibiting oxidation of the His residue involved in the AT-band formation. The efficiency of the AT-band quenching induced by diphenylcarbazide and hydroxylamine is almost an order of magnitude higher than the quenching efficiency of Mn(II) and Fe(II). Our results suggest that the redox-active His is not a ligand of the high-affinity site and does not participate in the electron transport from Mn(II) and Fe(II) to YZ. The concentration dependences of the AT-band inhibition by Mn(II) and Fe(II) coincide with each other, thereby implying specific interaction of Fe(II) with the donor side of PS II.
KEY WORDS: photosynthesis, photosystem II, water-oxidizing complex, manganese cluster, iron, thermoluminescence
Water oxidation in photosystem II (PS II) of higher plants, algae, and cyanobacteria takes place in oxygen-evolving complexes (OEC) [1-3]. A cluster of four manganese atoms plays a key role in the functioning of the OEC. This cluster mediates the cycle of photoinduced redox reactions of oxygen evolution [4]. It is well known that the Mn-cluster is attached to the inner side of the thylakoid membrane near the C-terminal domains of polypeptides D1 and D2 of the PS II reaction center (RC) complex. The results of extended X-ray absorption fine structure (EXAFS) structural studies revealed that the coordination sphere of the Mn-cluster contains nitrogen and oxygen atoms. Analysis of the Mn-binding sites in the Mn-containing proteins of known three-dimensional structure shows that histidine residues and carboxyl groups of glutamic and aspartic acids are involved in binding to the manganese cations. This conclusion was supported in experiments with chemical modification of amino acid residues constituting the Mn-binding sites of PS II [5-11]. Studies of mutant cyanobacteria suggested that the binding site of the Mn-cluster might include the following amino acid residues: His190 [12], His332, His337, Asp170, Asp342, and Glu333 [13, 14]. In addition, the Mn-binding site may incorporate the carboxyl groups of Ala344 of polypeptide D1 [15] and Glu69 of polypeptide D2 [16]. Electron spin-echo envelope modulation (ESEEM) studies also confirmed that the coordination sphere of the Mn-cluster contains histidine residues [17].
Although PS II complexes subjected to Mn extraction are unable to oxidize water, they are capable of mediating photoinduced oxidation of various exogenous electron donors: diphenylcarbazide (DPC), hydroxylamine (HA), Mn(II), Fe(II), and I- [18, 19]. It was suggested that oxidation of Mn by the tyrosine residue YZ takes place at the native segment(s) of the cation-binding site. The cation Mn(II) binds specifically to the high-affinity Mn-binding site [20]. Bound Mn(II) is oxidized by oxidized tyrosine YZox, thereby placing a limitation on the accessibility of YZox for exogenous donors (DPC and Mn(II)) [7]. This is accompanied by a decrease in the rate of photoinduced reduction of the exogenous electron donor 2,6-dichlorophenolindophenol (DCPIP) [6, 7, 19, 21]. This effect (the DPC/DCPIP-test) was used by some researchers to study the mechanisms of interaction of manganese cations and cations of other metals with the high-affinity Mn-binding site of the OEC [6, 19, 21, 22].
Thermoluminescence can be used to study of the electron transport chain of PS II [23]. The B- and Q-bands of thermoluminescence of intact PS II particles represent charge recombination between oxidized Mn-cluster in the S2- and S3-states and reduced electron acceptors QA and QB. Extraction of Mn is accompanied by disappearance of the main thermoluminescence bands and appearance of an additional thermoluminescence band, AT, which is due to charge recombination between the reduced primary acceptor QA- and an oxidized electron carrier at the donor side of PS II. Experiments with modification of histidine and carboxyl groups revealed that redox-active histidine is the electron carrier at the donor side of PS II responsible for the thermoluminescence band AT [24]. It was suggested that this histidine is involved in the binding of the Mn-cluster [24].
The DPC/DCPIP-test showed that Fe(II) effectively bind to the high-affinity Mn-binding site of PS II [19]. Certain characteristics of Fe(II) binding to the high-affinity Mn-binding site are similar to the characteristics of the binding of Mn(II) to this site. A kinetic study revealed that interaction of Fe(II) with the donor side of PS II is accompanied by formation of a two-atom center [25]. It was also shown that the two-atom center is possibly bound to amino acid residues of the C-terminal domains of polypeptides D1 and D2 of the PS II RC complex [26]. There is also experimental evidence that Fe(II) can bind to the high-affinity Mn-binding site involved in electron transfer from Mn(II) to YZox [19, 20, 25].
The goal of this work was to study the possible contribution of redox-active His to binding to Fe(II) and Mn(II). This study was performed by comparing the effects of Fe(II) and Mn(II) on the AT-band of thermoluminescence in Mn-free membrane preparations of PS II.
MATERIALS AND METHODS
Spinach PS II membrane preparations (BBY-particles of PS II) were prepared as described earlier [27]. The spinach was purchased in a local market. Photochemical activity of PS II particles was probed by the rate of O2 evolution as measured polarographically using a closed Clark Pt-electrode at +20°C. Ferricyanide (2 mM) and 2,6-dimethylbenzoquinone (2 mM) were used as exogenous electron acceptors. The mean photochemical activity of the BBY-particles was 500 µmol O2 per h per mg chlorophyll (Chl). Isolated preparations were suspended in buffer solution containing 400 mM sucrose, 15 mM NaCl, and 50 mM MES-NaOH (pH 6.1) (buffer A) and stored in liquid nitrogen until use.
Manganese was extracted by treatment of PS II particles with 0.8 M Tris-HCl buffer (pH 8.5) for 15 min at room temperature and exposure to scattered natural daylight. Treated particles were washed twice with buffer A. The residual oxygen-evolving activity of the Mn-free PS II particles (PS II (-Mn)) was ~15 µmol O2 per h per mg Chl.
The rate of electron transport in PS II particles was measured spectrophotometrically by the rate of photoinduced reduction of DCPIP (40 µM) using DPC (200 µM) as an exogenous electron donor. The extinction coefficient of deprotonated DCPIP, epsilon600, was taken to be 21 mM-1·cm-1. The concentration of Chl in all samples was 20 µg/ml. Only freshly prepared solutions of DPC, MnCl2, and FeSO4 were used.
The intensity of the thermoluminescence bands in PS II particles was measured using the luminometer developed at the Department of Biophysics, School of Physics, Lomonosov Moscow State University [28]. The procedure of measurement included the following stages: a 100-µl sample of suspension of PS II particles in buffer A (200 µg Chl per ml) was applied to a filter paper support, incubated in the dark for 1 min at 0°C, illuminated with a KGM-30-300 incandescent lamp (light intensity, 15 W/m2) at -30°C for 3 min (the temperature and light exposure time were optimal for detecting the B- and Q-bands of thermoluminescence), and rapidly cooled to -60°C. Thermoluminescence was recorded upon sample heating at a rate of 30°/min over the temperature range from -60 to +80°C.
Sucrose, DPC, and MES were from Sigma (USA). The other reagents were chemical (or higher than chemical) purity grade products of domestic manufacturers.
RESULTS
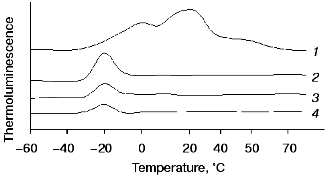
Effects of exogenous cations of manganese and iron on thermoluminescence of manganese-free PS II particles. Typical curves of thermoluminescence emission by PS II particles containing functionally active OEC, Mn-free PS II (PS II(-Mn)), and PS II (-Mn) in the presence of exogenous Mn(II) or Fe(II) are shown in Fig. 1, curves 1-4, respectively. Two thermoluminescence bands with maximums at +5 and +30°C were observed in intact PS II particles (Fig. 1, curve 1). These maximums are typical of the thermoluminescence bands B and Q, which are usually attributed to charge recombination between oxidized Mn-cluster in S3- or S2-states and reduced electron acceptors QA- or QB-, respectively [23]. Extraction of Mn from the OEC is accompanied by disappearance of these bands and simultaneous appearance of an additional thermoluminescence band at -18°C (the so-called AT-band [24]). Figure 1, curve 3, shows that addition of 15 µM MnCl2 to PS II (-Mn) preparations causes a decrease in the amplitude and area of the AT-band. Addition of Fe(II) had a similar effect on the AT-band of thermoluminescence (Fig. 1, curve 4).
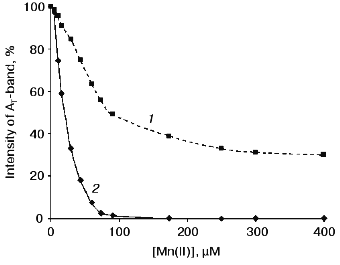
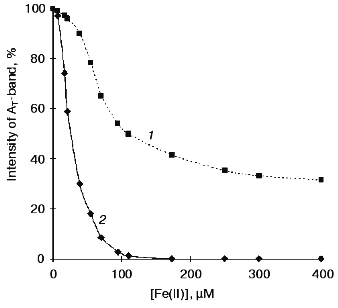
The curves of concentration dependence of quenching of the thermoluminescence AT-band of Mn-free PS II particles by exogenous Mn(II) and Fe(II) as added immediately before measurements are shown in Figs. 2 and 3. These figures show that both Mn(II) and Fe(II) induce a decrease in the amplitude of the AT-band of thermoluminescence, the quenching efficiencies of the two cations being approximately equal. A 50% decrease in the amplitude of the AT-band of thermoluminescence was observed in the presence of 90 and 120 µM of Mn(II) and Fe(II), respectively. These concentrations corresponded to Mn/RC and Fe/RC ratios of 90 : 1 and 120 : 1, respectively (assuming 200 Chl molecules are associated with each RC complex). The maximum efficiency of quenching of the thermoluminescence AT-band (70%) was observed in the presence of 300 µM Mn(II) or 400 µM Fe(II).Fig. 1. Thermoluminescence of intact PS II particles (1), Mn-free PS II particles [PS II (-Mn)] (2), and effect of exogenous Mn(II) (3) and Fe(II) (4) on the thermoluminescence of PS II (-Mn) particles. The concentration of MnCl2 and FeSO4 in experimental samples was 15 µM.
Fig. 2. Concentration dependence of quenching of the thermoluminescence AT-band of Mn-free PS II particles by exogenous Mn(II): 1) Mn(II) were added to the suspension of PS II (-Mn) particles immediately before thermoluminescence measurement; 2) thermoluminescence was measured after preliminary incubation (3 min, room temperature, exposure to scattered natural daylight) of PS II (-Mn) particles in the presence of Mn(II).
Effect of preliminary exposure to light on the efficiency of quenching of the thermoluminescence AT-band by Mn(II) and Fe(II). It is known that short-term (3-5 min) exposure of PS II (-Mn) particles to scattered daylight in the presence of Fe(II) is accompanied by irreversible binding of Fe(II) to the high-affinity Mn-binding site of PS II [19, 25]. According to the literature, the redox-active histidine involved in formation of the thermoluminescence AT-band is incorporated in the coordination sphere of the Mn-cluster of PS II [24]. Therefore, the problem of functional connection between His+ induction and binding of exogenous cations to the high-affinity Mn-binding site is of considerable interest. In further experiments, we studied the effect of preliminary light adaptation of PS II (-Mn) preparations in the presence of Mn(II) and Fe(II) on the efficiency of quenching of the thermoluminescence AT-band by these cations. Concentrations of Mn(II) and Fe(II) used in these experiments are given in legends to Fig. 2 (curve 2) and Fig. 3 (curve 2), respectively. Figures 2 and 3 show that preliminary exposure to scattered daylight provides binding of the exogenous cations to the high-affinity Mn-binding site and significant decrease in the amplitude of the thermoluminescence AT-band. Complete disappearance of the thermoluminescence AT-band in preliminarily illuminated PS II particles was observed in the presence of substantially lower concentrations of Mn(II) and Fe(II) than in the absence of preliminary illumination. A 50% decrease in the amplitude of the AT-band of thermoluminescence in the preliminarily illuminated PS II particles was observed in the presence of 25 and 30 µM of Mn(II) and Fe(II), respectively. These concentrations corresponded to Mn/RC and Fe/RC ratios of 25 : 1 and 30 : 1, respectively. An increase in the concentration of either of the cations to 80 µM caused complete disappearance of the thermoluminescence AT-band.Fig. 3. Concentration dependence of quenching of the thermoluminescence AT-band of Mn-free PS II particles by exogenous Fe(II): 1) Fe(II) was added to the suspension of PS II (-Mn) particles immediately before thermoluminescence measurement; 2) thermoluminescence was measured after preliminary incubation (3 min, room temperature, exposure to scattered natural daylight) of PS II (-Mn) particles in the presence of Fe(II).
It is well known that exposure of Mn-free PS II particles to scattered daylight induces the process of donor photoinhibition. This is accompanied by degradation of the high-affinity Mn-binding site, whereas exogenous electron donors (e.g., Mn(II)) prevent this site from the photoinhibition-induced degradation [18]. However, the effect observed in our experiments cannot be attributed to donor photoinhibition because the amplitude of the thermoluminescence AT-band in Mn-free PS II particles subjected to preliminary illumination in the absence of exogenous ions was no more than 8% smaller than in control preparations. Obviously, in the presence of electron donors (Mn(II) and Fe(II)), the effect of photoinhibition is expected to be significantly suppressed.
Perhaps, complete inhibition of the thermoluminescence AT-band by Mn(II) and Fe(II) after preliminary light incubation of PS II (-Mn) particles can be regarded as evidence of either more effective donation of electrons to His+ or hindered induction of His+ itself upon Mn/Fe binding to the high-affinity Mn-binding site.
DPC/DCPIP-test for Mn(II) and Fe(II) binding to the high-affinity Mn-binding site. The processes of binding of the Mn(II) and Fe(II) to the high-affinity Mn-binding site of Mn-free preparations of PS II were studied to elucidate the contribution of the His residue, responsible for the AT-band, to the binding process. According to the literature [6, 21], the binding of Mn to the high-affinity site can be monitored by measuring the rate of electron transport from DPC to DCPIP. In the absence of Mn, DPC is able to donate electrons to two carriers (YZox and YDox). Binding of Mn cations to the high-affinity Mn-binding site results in inhibition of electron donation from DPC to YZox, thereby causing a twofold decrease in the overall rate of electron transport.
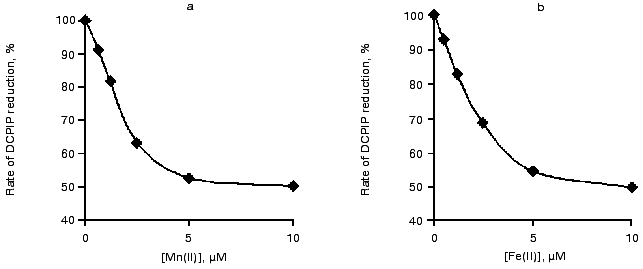
The curves of concentration dependence of the efficiency of binding of Mn(II) and Fe(II) to the high-affinity Mn-binding site during exposure to light are shown in Fig. 4. This figure shows that complete binding of the added cations to the high-affinity binding site (50% decrease in the rate of electron transfer from DPC to DCPIP) is observed in the presence of virtually equal concentrations of Mn(II) and Fe(II) (1.5 or 1.7 µM, respectively). These values correspond to the Mn/RC and Fe/RC ratio of 15 : 1 and 17 : 1, respectively.
Effect of DPC and HA on the thermoluminescence AT-band. Direct reduction of His+ at the donor side of PS II can be regarded as a possible cause of quenching of the thermoluminescence AT-band by exogenous Mn(II) and Fe(II). To test this suggestion, we studied the effects of other electron donors for PS II, DPC and HA, on the thermoluminescence AT-band.Fig. 4. Effect of Mn(II) (a) and Fe(II) (b) on the rate of electron transfer along the chain of carriers: DPC --> PS II (-Mn) --> DCPIP. Concentrations of components: Chl, 20 µg/ml; DPC, 200 µM; and DCPIP, 40 µM.
The efficiency of quenching of the AT-band induced by DPC or HA was found to be almost an order of magnitude higher than the quenching efficiency of Mn(II) or Fe(II). It should also be noted that although the addition of Mn(II) or Fe(II) caused only partial inhibition of the thermoluminescence AT-band, this band disappeared completely in the presence of only 30 molecules of DPC per RC or 10 molecules of HA per RC (table).
Effect of exogenous electron donors on the thermoluminescence
AT-band
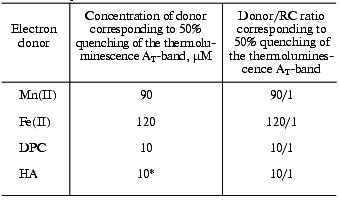
*Concentration corresponding to 100% quenching of the
AT-band.
DISCUSSION
According to the literature, a large body of experimental data shows that the coordination sphere of the Mn-cluster of PS II contains histidine residues. For example, quite convincing and direct evidence was obtained in an ESEEM study of PS II reaction centers with native protein nitrogen substituted by the 15N-isotope [17]. Site-directed mutagenesis revealed that substitution of the His190, His332, and His337 residues in the integral RC protein D1 by other amino acid residues has a significant effect on the functional activity of the Mn-cluster. These histidine residues are considered as the most probable ligands of Mn [12-14]. His332 and His337 are thought to be bound to Mn, whereas His190 seems to play a more intricate role because substitution of this residue exerts a significant effect on the redox properties of YZ [29]. The thermoluminescence studies of mutants with substituted residues His190 and His195 showed that phenylalanine substitution for His190 is accompanied by complete disappearance of the AT-band [30], which is attributed to charge recombination between QA- and His+ at the donor side of PS II [24]. However, site-directed mutagenesis allows only the suggestion that His190 is responsible for the AT-band formation because replacement of even a single amino acid residue can cause significant changes in protein molecule structure [30]. The donor side of PS II contains four Mn-binding sites. One of these sites (the high-affinity site) has been studied rather comprehensively, but it remains uncertain if His is a ligand of the high-affinity Mn-binding site. Although the data concerning this problem are quite controversial, there is a large body of evidence that His is not incorporated in the coordination sphere of Mn bound to the high-affinity Mn-binding site [7, 11]. For example, it was shown in [11] that chemical modification of histidine has no effect on the efficiency of electron donation from manganese to YZ through the high-affinity site under condition of photoactivation with the first light flash.
The effect of partial (70%) quenching of the thermoluminescence AT-band induced in PS II (-Mn) particles by exogenous Mn(II) (Fig. 2) is consistent with previous findings [31, 32]. According to the results reported in [31, 32], only 60% inhibition of the thermoluminescence AT-band was observed in the presence of 1 mM Mn(II). These findings can be regarded as evidence the His residue responsible for formation of the thermoluminescence AT-band is not incorporated in the high-affinity binding site mediating electron transfer from Mn to YZox. Indeed, even in the absence of actinic light exogenous Mn(II) binds specifically to PS II (-Mn). Moreover, the stoichiometric ratio 1 Mn(II)/1 RC was found to be sufficient to provide complete reduction of YZox photooxidized by a light flash [20]. In our experiments, the addition of Mn(II) at stoichiometric ratio 90 Mn(II)/1 RC PS II caused only 50% inhibition of the thermoluminescence AT-band, whereas the ratio measured in the DPC/DCPIP-test of Mn binding to the high-affinity binding site (electron transfer from DPC to DCPIP) was found to be six times lower. We also found that the quenching efficiency of DPC, an electron donor for YZox, with respect to the thermoluminescence AT-band was almost an order of magnitude higher than the quenching efficiency of Mn(II). It was shown in [11] that modification of His residues more significantly inhibits electron transfer from DPC to Mn-free PS II than electron transfer from Mn(II) to Mn-free PS II. Because electron transfer from DPC or Mn(II) to YZox is mediated by different sites [6], the His+ residue responsible for the AT-band is located at the electron transport chain segment involving DPC rather than Mn. This can be regarded as additional evidence that His is not a ligand of the high-affinity Mn-binding site.
Instead of the sequential model suggested by Ono and Inoue [24] (His --> YZ --> P-680), a parallel model of P-680+ reduction was put forward in [30]. According to this model, an electron is transported from His to P-680 only at low temperature. Perhaps, at the illumination temperature of -30°C DPC is able to donate electrons directly to His+, whereas at room temperature DPC reduces P-680+ as a result of electron donation through YZox [22].
Exposure of PS II (-Mn) preparations to light in the presence of exogenous Mn(II) at concentration ratio Mn(II)/RC PS II = 15 : 1 induces manganese oxidation and Mn(III) binding to the high-affinity binding site (according to the DPC/DCPIP-test). Presumably, this binding is accompanied by structural disengagement between His and P-680, thereby preventing oxidation of His. As a result, induction of the thermoluminescence AT-band is completely inhibited. In our experiments such inhibition was observed within the same manganese concentration range as its binding to the high-affinity binding site. Because this effect would be absent if Mn(II) were added immediately before thermoluminescence detection without preliminary illumination, the temperature dependence of manganese oxidation and/or binding to the high-affinity binding site is thought to be strong. For example, the rate of electron transport at the acceptor side of PS II decreases at temperatures below -30°C [33], whereas formation of the S3 and S4 states is inhibited at temperatures below -35°C [34]. It should also be noted that His190, a hypothetical electron donor for P-680+, at temperatures below 0°C is located near the tyrosine YZ. There is a network of hydrogen bonds or water molecule bridges between the tyrosine YZ and His190 under these conditions [35]. Some researchers suggest that the functional role of His190 is to accept a proton during oxidation of YZ [29, 35]. It is also conceivable that Mn(II) binding in the light at room temperature affects the tyrosine YZ and modifies protonation of His190. This may also modify the mutual structural arrangement of His190 and P-680.
The effects of Fe(II) on the thermoluminescence AT-band of PS II (-Mn) shown in this work is similar to the effect induced by the Mn(II). Moreover, it was found that the concentrations of the cations causing 50% quenching of the thermoluminescence AT-band under conditions of preliminary exposure of PS II (-Mn) preparations to light are quite similar (the cation/RC ratios for Mn(II) and Fe(II) are 25 : 1 and 30 : 1, respectively). The affinity values of these cations for the high-affinity Mn-binding site are also similar: 15 Mn(III)/RC and 17 Fe(III)/RC. It was shown in the preceding works [19, 26] that Fe(II), like Mn(II), is capable of specific binding to the high-affinity binding site of PS II (-Mn) preparations. The results obtained in this work are consistent with these findings and show that photoinduced oxidation of Fe(II) is accompanied by its binding to the high-affinity Mn-binding site in the PS II OEC.
This study was supported by the Russian Foundation for Basic Research, project No. 99-04-48881.
REFERENCES
1.Nugent, J. H. A. (1996) Eur. J. Biochem.,
237, 519-531.
2.Renger, G. (1997) Physiol. Plant.,
100, 828-841.
3.Hankamer, B., and Barber, J. (1997) Annu. Rev.
Plant Physiol. Plant Mol. Biol., 8, 641-671.
4.Kok, B., Forbush, B., and McGloin, M. (1970)
Photochem. Photobiol., 11, 457-475.
5.Tamura, N., Ikeuchi, M., and Inoue, Y. (1989)
Biochim. Biophys. Acta, 973, 281-289.
6.Preston, C., and Seibert, M. (1991)
Biochemistry, 30, 9615-9624.
7.Blubaugh, D. J., and Cheniae, G. M. (1992)
Research in Photosynthesis, Vol. 2, Kluwer Academic Publishers,
Dordrecht, The Netherlands, pp. 361-364.
8.Magnuson, A., and Andreasson, L.-E. (1997)
Biochemistry, 36, 3254-3261.
9.Tamura, N., Noda, K., Wakamatsu, K., Kamachi, H.,
Inoue, H., and Wada, K. (1997) Plant Cell Physiol., 38,
578-585.
10.Ghirardi, M. L., Lutton, T. W., and Seibert, M.
(1998) Biochemistry, 37, 13559-13566.
11.Ghirardi, M. L., Lutton, T. W., and Seibert, M.
(1998) Biochemistry, 37, 13567-13574.
12.Chu, H. A., Nguyuen, A. P., and Debus, R. J.
(1995) Biochemistry, 34, 5839-5858.
13.Chu, H. A., Nguyuen, A. P., and Debus, R. J.
(1995) Biochemistry, 34, 5859-5882.
14.Boerner, R. J., Nguyen, A. P., Barry, B. A., and
Debus, R. J. (1992) Biochemistry, 31, 6660-6672.
15.Nixon, P. J., and Diner, B. A. (1992)
Biochemistry, 31, 19859-19871.
16.Vermaas, W. F. J., Charite, J., and Shen, G.
(1990) Biochemistry, 29, 5325-5332.
17.Tang, X.-S., Diner, B. A., Larsen, B. S.,
Gilchrist, M. L., Lorigan, G. A., and Britt, R. D. (1994) Proc.
Natl. Acad. Sci. USA, 91, 704-708.
18.Blubaugh, D. J., and Cheniae, G. M. (1990)
Biochemistry, 29, 5109-5118.
19.Semin, B. K., Ivanov, I. I., Rubin, A. B., and
Parak, F. (1995) FEBS Lett., 375, 223-226.
20.Ono, T., and Mino, H. (1999) Biochemistry,
38, 8778-8785.
21.Hsu, B.-D., Lee, J.-I., and Pan, R. L. (1987)
Biochim. Biophys. Acta, 890, 89-96.
22.Ghirardi, M. L., Lutton, T. W., and Seibert, M.
(1996) Biochemistry, 35, 1820-1828.
23.Govindjee (1996) Photosynthesis Res.,
48, 117-126.
24.Ono, T., and Inoue, Y. (1991) FEBS Lett.,
278, 183-186.
25.Semin, B. K., Davletshina, L. N., Ivanov, I. I.,
Reiner, M., and Parak, F. (1998) in Photosynthesis: Mechanisms and
Effects (Garab, G., ed.) Vol. 2, Kluwer Academic Publishers,
Dordrecht, The Netherlands, pp. 1415-1418.
26.Semin, B. K., and Parak, F. (1997) FEBS
Lett., 400, 259-262.
27.Berthold, D. A., Babcock, G. T., and Yocum, C. F.
(1981) FEBS Lett., 134, 231-234.
28.Solntsev, M. K., Ekobena, P. F., Karavaev, V. A.,
and Yurina, T. P. (1998) J. Luminescence, 76,
349-353.
29.Mamedov, F., Sayre, R. T., and Styring, S. (1998)
Biochemistry, 37, 14245-14256.
30.Kramer, D. M., Roffey, R. A., Govindjee, and
Sayre, R. T. (1994) Biochim. Biophys. Acta, 1185,
228-237.
31.Tamura, N., Noda, K., Wakamatsu, K., Kamachi, H.,
Inoue, H., and Wada, K. (1997) Plant Cell Physiol., 38,
578-585.
32.Ono, T., and Inoue, Y. (1991)
Biochemistry, 30, 6183-6188.
33.Joliot, A. (1974) Biochim. Biophys. Acta,
357, 439-448.
34.Inoue, Y., and Shibata, K. (1978) FEBS
Lett., 85, 193-197.
35.Hays, A. N., Vassiliev, I. R., Golbeck, J. H.,
and Debus, R. J. (1999) Biochemistry, 38,
11851-11865.