Cationic Inhibitors of Serine Proteinases from Buckwheat Seeds
T. A. Tsybina1, Y. E. Dunaevsky1, A. Kh. Musolyamov2, T. A. Egorov2, and M. A. Belozersky1*
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3181; E-mail: mbeloz@belozersky.msu.ru2Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, ul. Miklukho-Maklaya 16/10, Moscow, 117871 Russia; fax: (095) 330-7301; E-mail: ego@bch.siobc.ras.ru
* To whom correspondence should be addressed.
Received May 14, 2001
Preparations of low molecular weight protein inhibitors of serine proteinases have been obtained from buckwheat (Fagopyrum esculentum) seeds by chromatography of seed extract on trypsin-Sepharose 4B, Mono-Q, and Mono-S ion exchangers (FPLC regime). Their molecular masses, determined by mass spectrometry, were 5203 (BWI-1c), 5347 (BWI-2c), 7760 (BWI-3c), and 6031 daltons (BWI-4c). All of the inhibitors possess high pH- and thermal stability in the pH range 2-12. In addition to trypsin, BWI-3c and BWI-4c inhibited chymotrypsin and subtilisin-like bacterial proteases. The N-terminal sequences of all of the inhibitors were determined: BWI-1c (23 residues), BWI-2c (33 residues), BWI-3c (18 residues), and BWI-4c (20 residues). In their physicochemical properties and N-terminal amino acid sequences, the buckwheat seed trypsin inhibitors BWI-3c and BWI-4c appear to belong to potato proteinase inhibitor I family.
KEY WORDS: serine proteinase inhibitors, trypsin, subtilisin, chymotrypsin
Abbreviations: Bz) benzoyl; Glp) pyroglutamyl; Z) N-carbobenzoxy; pNA) p-nitroanilide; BWI) serine proteinase inhibitors from buckwheat seeds.
Protein serine proteinase inhibitors are widely distributed in plants
and are obtained from many sources [1]. Seeds are
especially rich in inhibitors. The classification of inhibitors differs
in the works of various authors; the classifications are based on
homology of amino acid sequences, active center structure, position of
disulfide bonds, and mechanisms of inhibition [1,
2].
Most of plant proteinase inhibitors that are known and studied so far interact with serine proteinases (trypsin, chymotrypsin, subtilisin). In recent years, protein inhibitors of cysteine proteinases have also been isolated and characterized [3]. Protein inhibitors of aspartic and metalloproteinases remain nearly unstudied.
Although the biological role of protein proteinase inhibitors is not still sufficiently clear, it has been suggested that they may perform three main functions--serving as storage proteins, being regulators of activity of endogenous proteinases and agents protecting plants against insects and pathogenic microflora [4].
When this work was begun, data already existed indicating the presence in buckwheat seeds of trypsin inhibitors that suppress spore germination and mycelium growth of the fungus Alternaria alternata [5]. Investigation of these inhibitors revealed among them the presence of two groups, differing in behavior on ion-exchange chromatography and in isoelectric points (pI): anionic and cationic protease inhibitors. Protease inhibitors from buckwheat seeds with lower pI values, considered as anionic, were described earlier [6].
This work presents results on isolation from dry buckwheat seeds of cationic protease inhibitors and study of their physicochemical properties and amino acid sequences.
MATERIALS AND METHODS
Plant material. Dry buckwheat (Fagopyrum esculentum Moench cv. Shatilovskaya-5) seeds were used.
Inhibitor activity was evaluated according to the degree of suppression of activities of corresponding enzymes (trypsin, alpha-chymotrypsin, subtilisin-72 from B. subtilis, trypsin- and subtilisin-like enzymes from fungi). Solution containing a mixture of inhibitor and enzyme was incubated at room temperature for 10 min. The residual enzyme activity was assayed according to Erlanger et al. [7] after 30 min at 37°C using as substrates 0.6 mM Bz-Arg-pNA in 0.1 M K,Na-phosphate, pH 7.0 (for trypsin), 0.6 mM Glp-Phe-pNA (for alpha-chymotrypsin), 5 mM Z-Ala-Ala-Leu-pNA and Glp-Ala-Ala-Leu-pNA (for subtilisin-like enzymes) in dimethylformamide, which were diluted before use with 0.05 M K,Na-phosphate, pH 8.0 (1 : 10). The reaction was stopped by addition of acetic acid to the incubation mixture to 5% final concentration.
One unit of inhibitor activity corresponded to its amount that produced a decrease in absorption at 410 nm (A410) by 0.1 unit in the inhibitor activity assay at 50% inhibition. In case of assay of inhibitor activity towards partially purified preparations of fungal proteinases, the enzyme activity was compared to the activity of highly purified preparations of trypsin and subtilisin using concentrations of these enzymes in calculations.
Protein concentration in inhibitor solutions was assayed according to Lowry et al. [8] and spectrophotometrically at 280 nm. Protein solutions were concentrated by ultrafiltration using Amicon (The Netherlands) cells with YM-5 membranes.
PAGE analysis of inhibitors under non-denaturing conditions was carried out according to Davis [9] in 10% gel for 1 h at 600 V and 5 mA per tube. The gels were stained with 0.04% Coomassie G-250 in 4% hydrochloric acid, and excess dye was removed with 7% acetic acid.
Modification of amino acids. To determine the amino acids at the reactive site of inhibitor molecules, lysine residues were modified with acetic anhydride [10] and arginine residues with diacetyl (2,3-butanedione) [11].
Thermostability and pH-stability. The stability of inhibitors at different pH values was assayed using 0.05 M universal buffer in the pH 2.0-12.0 range (with 0.5 pH unit intervals). Inhibitor solution was incubated at different pH values at 4°C for 4 h. Then the pH of the mixture was brought to 7.0 with 0.25 M phosphate, pH 7.0, and inhibitor activity towards trypsin was assayed as described above. To study the thermostability, the inhibitor preparation was incubated at 100°C for 15 and 30 min at different pH values.
Amino acid composition of inhibitors was determined by a standard procedure with a Hitachi 835 (Japan) amino acid analyzer. To determine sulfur-containing amino acids, the protein was oxidized with a mixture of 30% H2O2 and 88% performic acid (1 : 9 v/v), followed by hydrolysis with 5.7 M HCl (110°C, 22 h) (cysteine was determined as cysteic acid). Tryptophan was determined after hydrolysis of the protein with 4 M methanesulfonic acid containing 0.2% tryptamine.
Reduction and alkylation of proteins was carried out with 4-vinylpyridine [12]. The alkylated protein was desalted by reversed-phase HPLC and evaporated in a Speedvac (Savant, USA) concentrator.
Automated Edman degradation. The N-terminal amino acid sequences of proteins were determined using a Model 816 Protein/Peptide Sequencer (Knauer, Germany), equipped with a Model 120A PTH-analyzer (Applied Biosystems). Preparations of protein and peptides were dissolved in 10-15 µl of 50% acetonitrile containing 0.1% TCA and applied in 5 µl portions to the Immobilone P membrane (Millipore, USA) for sequencing.
Molecular masses of inhibitors were determined with the time-of-flight Reflex II (Brucker-Franzen Analytik GmbH, Germany) mass spectrometer according to the protocols of the manufacturer.
RESULTS AND DISCUSSION
Cationic protease inhibitors, isolated from dry buckwheat seeds were studied in the present work. The developed isolation technique involved the following stages. Seeds were ground in an electric mill. The proteins were extracted with 0.1 M K,Na-phosphate, pH 6.8 (buffer A) (1 : 4 w/v) at 4°C for 18 h. The extract was centrifuged at 14,000g for 40 min at 5°C. Dry (NH4)2SO4 was added to the supernatant (to 80% saturation), and the precipitate was formed for 18 h at 4°C. The precipitate was centrifuged at 14,000g for 40 min at 5°C and dialyzed against buffer A (24 h, 4°C, double change of buffer). The denatured protein was separated by centrifugation at 18,000g for 25 min at 5°C. The fraction of soluble proteins was subjected to affinity chromatography on a trypsin-Sepharose 4B column synthesized using CNBr-activated Sepharose 4B [13].
The inhibitors were sorbed on the affinity column in buffer A containing 0.5 M NaCl at 4°C for 4 h. Unbound proteins were thoroughly washed off, and the inhibitors were eluted with 1 mM HCl, pH 2.7, containing 0.5 M NaCl.
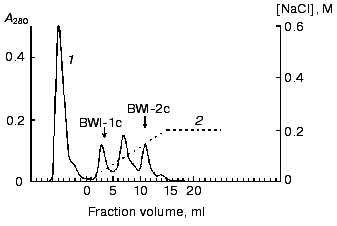
The resulting inhibitor fraction was concentrated and dialyzed against 10 mM K,Na-phosphate, pH 6.8 (buffer B). The total preparation of inhibitors (10 mg) was applied to an Mono-Q anion-exchange column (Pharmacia, Sweden) (FPLC regime) in buffer B, and the fraction of inhibitors not bound to the column under these conditions, was collected (the fraction of anionic inhibitors, which bind to the column, was investigated earlier [6]). This fraction was applied to a Mono-S cation-exchange column (FPLC regime) in buffer B. The fraction of unbound proteins was separated, and proteins sorbed on the ion exchanger were eluted with a linear NaCl gradient (0-0.2 M, 1 ml/min, 25 min). Two protein fractions (BWI-1c and BWI-2c) with trypsin inhibiting activity were found in the eluate (Fig. 1). The peak between these fractions contained a mixture of the two inhibitors.
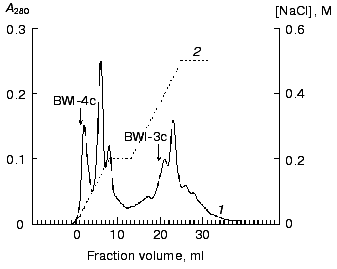
The non-sorbed proteins were concentrated and equilibrated with 0.1 M citrate-phosphate, pH 4.0 (buffer C), and further applied to a Mono-S column (FPLC regime) in buffer C. The sorbed proteins were eluted with a linear NaCl gradient (0-0.2 M, 10 min, 1 ml/min). Two protein fractions (BWI-3c and BWI-4c) possessing significant trypsin inhibiting activity were found in the eluate (Fig. 2). The yield and purification degree of each fraction are given in Table 1. The apparent decrease in specific activity and purification degree at the stage of Mono-Q column chromatography was due to separation of the total fraction of inhibitors into quantitatively predominating fractions of anionic inhibitors sorbed to the column under the mentioned conditions and studied earlier [6], and the fraction of cationic inhibitors with higher pI and not sorbed to the column.Fig. 1. Chromatography of cationic protease inhibitors from buckwheat seeds on Mono-S, pH 6.8: 1) A280; 2) NaCl concentration.
Table 1. Purification of cationic protease inhibitors from buckwheat seedsFig. 2. Chromatography of cationic protease inhibitors from buckwheat seeds on Mono-S, pH 4.0: 1) A280; 2) NaCl concentration.
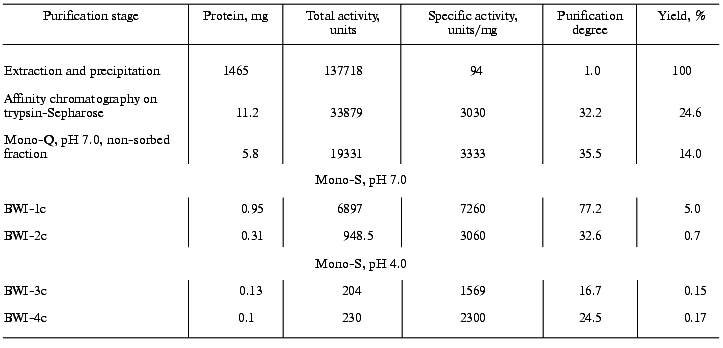
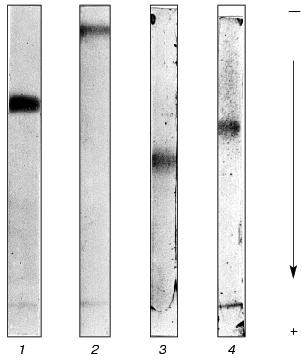
From this purification procedure, trypsin-inhibiting fractions BWI-1c-4c were obtained in electrophoretically homogeneous form (Fig. 3) and characterized.
Because proteolytic enzymes may be inactivated by non-proteinaceous substances present in plants, in particular by phenolic compounds [14] and polyamines [15], experiments were done to confirm the protein nature of the inhibitors. For this purpose, solution of the inhibitor (0.5-4.0 µg) was incubated for 24 h at 37°C in the presence of 3-fold excess of pepsin (calculated per mol) in 0.5 M citrate-phosphate, pH 2.7. The pH of the mixture was then brought to 7.8-8.0 by addition of 1 M Tris-HCl, pH 9.2, and the activity of the inhibitors towards trypsin, chymotrypsin, and subtilisin-72 was assayed. It was demonstrated that preparations of the inhibitors treated with pepsin lost their ability to inhibit trypsin, chymotrypsin, and subtilisin-72, whereas the control preparations completely retained activity after incubation at pH 2.7. Thus, the cationic inhibitors isolated from buckwheat seeds are proteins.Fig. 3. Electrophoretic pattern of purified cationic protease inhibitors from buckwheat seeds: BWI-1c (1), BWI-2c (2), BWI-3c (3), BWI-4c (4).
Table 2 presents data on the effects of the inhibitors on the activity of various proteolytic enzymes. The differing specificity of action of the studied inhibitors should be noted. Whereas inhibitors BWI-1c and BWI-2c inactivated trypsin, inhibitors BWI-3c and BWI-4c inactivated alpha-chymotrypsin and bacterial subtilisins. Inhibitors BWI-3c and BWI-4c also inactivated subtilisin-like enzymes--termitase and savinase used in production of washing materials.
Table 2. Effect of cationic protease
inhibitors from buckwheat seeds on the activities of various
proteolytic enzymes
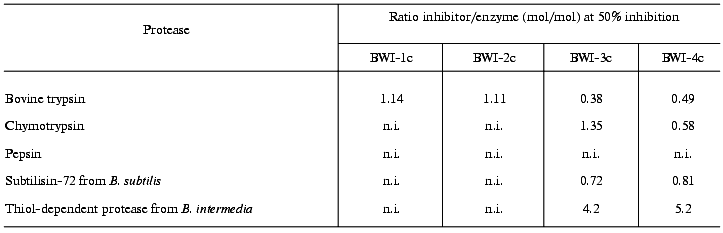
Note: n.i., no inhibition.
The molecular masses of the inhibitors determined by mass spectrometry were 5203 daltons for BWI-1c, 5347 daltons for BWI-2c, 7760 daltons for BWI-3c, and 6031 daltons for BWI-4c, these values being less than the molecular masses of anionic inhibitors (7.5-7.7 kD). The isoelectric points of all of the cationic inhibitors were in the pH range 8.2-8.7.
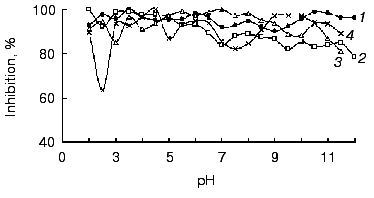
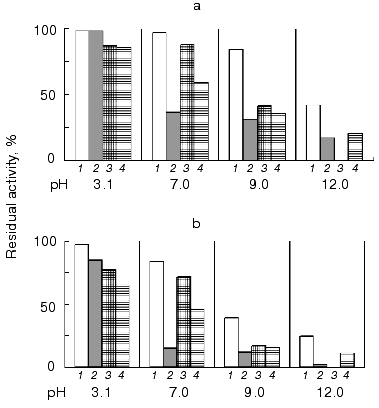
All of the studied inhibitors were highly stable to pH in the range 2.0-12.0 (Fig. 4), this being consistent with data on other low molecular mass inhibitors of proteolytic enzymes [16, 17]. A small decrease in the inhibitor activity (not more than 20%) was observed in the alkaline pH region, and for inhibitors BWI-3c and BWI-4c at pH lower than 4.0. Study of temperature stability of the inhibitors at different pH values revealed that the inhibitors were highly stable during incubation at 100°C for 30 min at acid pH values (Fig. 5). High thermostability, particularly in acid medium, is characteristic of inhibitors of the potato proteinase inhibitor I and II families. However there are inhibitors more stable to heating in alkaline medium, such as trypsin and chymotrypsin inhibitor from amaranth seeds [18] and subtilisin inhibitor from adzuki beans [19]. The relative stability of the inhibitor BWI-1c under neutral and alkaline conditions in comparison to other protease inhibitors from buckwheat seeds should be noted.
Fig. 4. Stability versus pH of cationic protease inhibitors from buckwheat seeds: BWI-1c (1), BWI-2c (2), BWI-3c (3), BWI-4c (4).
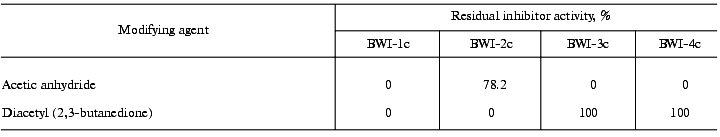
To study the nature of the amino acid residues at the reactive sites of the inhibitors, the effect of reagents modifying specific amino acid residues on the activity of the inhibitors was investigated. The data are summarized in Table 3. The data indicate that the reactive site of inhibitors BWI-3c and BWI-4c contains a lysine residue, and that of BWI-2c an arginine residue. Concerning inhibitor BWI-1c, the loss of its activity after modification of both lysine and arginine residues may be due to a change in the conformation of the inhibitor molecules during binding of the modifying agents. Alternatively, one of the modified residues, though not being involved in the reactive site of the inhibitor, may disturb the interaction of the inhibitor with the target enzyme.Fig. 5. Residual activity of the purified cationic protease inhibitors from buckwheat seeds after boiling for 15 (a) and 30 min (b) at different pH values: BWI-1c (1), BWI-2c (2), BWI-3c (3), BWI-4c (4).
Table 3. Modification of arginine and lysine
residues in the cationic protease inhibitors from buckwheat seeds
The study of amino acid composition of the buckwheat seed cationic protease inhibitors (Table 4) showed that all of the inhibitors contained a large number of glutamine and glutamic acid residues. There are no free SH-groups in the inhibitor molecules, all cysteine residues forming disulfide bonds. Inhibitors BWI-3c and BWI-4c have a high content of glycine, valine, and arginine residues as demonstrated also for other inhibitors isolated from buckwheat seeds. In contrast to buckwheat seed trypsin inhibitors isolated by other authors [6, 20, 21], inhibitors BWI-1c and BWI-2c contained fewer proline and arginine residues, and inhibitors BWI-3c and BWI-4c contained more of threonine and alanine residues.
Table 4. Amino acid composition of the
buckwheat seed protease inhibitors
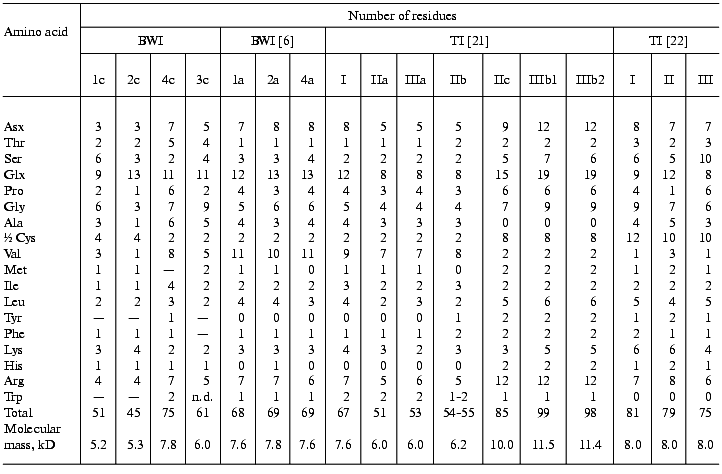
Note: TI, trypsin inhibitor; n.d., not determined.
Inhibitors BWI-3c and BWI-4c had noticeable homology in amino acid sequences of the N-terminal fragments with the buckwheat anionic protease inhibitors studied earlier [22, 23]. Analysis of the N-terminal amino acid sequences of the studied inhibitors (Fig. 6) pointed to a high degree of homology between them and proteins of the potato proteinase inhibitor I family. The degree of homology with the fragment of the N-terminal sequence of inhibitor AmTI from amaranth seeds was 55.5% for BWI-3c and 45% for BWI-4c. All inhibitors of the mentioned family showed conservatism at positions Lys8, Pro12-Glu13-Leu14, and Gly16. Positions Cys5, Gly7, and Val15 were relatively stable. Lys8 and Trp11 residues, present in nearly all of the inhibitors compared, were replaced in the BWI-4c molecule by Leu8 and Glu11, respectively.
The N-terminal sequences of BWI-1c and BWI-2c are highly homologous with each other and with trypsin inhibitor BWI-2b studied by Japanese workers [26]. According to their sequences, these inhibitors cannot be assigned to any presently known families of inhibitors of proteolytic enzymes.Fig. 6. N-Terminal amino acid sequences of buckwheat seed protease inhibitors and inhibitors of the potato proteinase inhibitor I family: BWI-1a, BWI-2a, and BWI-4a) anionic protease inhibitors from dry buckwheat seeds [22, 23]; AmTI) trypsin inhibitor from amaranth (Amaranthus hypochondriacus) seeds [24]; PI-IA) the protomer of inhibitor I from potato tubers (var. Russet Burbank) [25]; BWI-2b) trypsin inhibitor from buckwheat seeds [26].
Thus, four new, low-molecular-mass inhibitors of serine proteinases were isolated from the seeds of buckwheat and characterized. All of the inhibitors possessed high pH- and thermostability in acidic medium. Whereas inhibitors BWI-1c and BWI-2c were active only towards trypsin, inhibitors BWI-3c and BWI-4c effectively suppressed the activity of trypsin, chymotrypsin, and subtilisin-72 from B. subtilis. The data on the physicochemical properties and amino acid sequences suggest that BWI-3c and BWI-4c inhibitors belong to the potato proteinase inhibitor I family.
This work was supported by grants from the Russian Foundation for Basic Research (grant 00-04-48314) and the State Program of Russia “Phytobiotechnology”.
REFERENCES
1.Mosolov, V. V., and Valueva, T. A. (1993) Plant
Protein Inhibitors of Proteolytic Enzymes [in Russian], VINITI,
Moscow.
2.Laskowski, M., Jr., and Kato, I. (1980) Ann.
Rev. Biochem., 49, 593-626.
3.Pernas, M., Sánches-Monge, R., and Salcedo,
G. (2000) FEBS Lett., 467, 206-210.
4.Ryan, C. A. (1981) in The Biochemistry of
Plants (Marcus, A., ed.) Vol. 6,Academic Press, N. Y., pp.
351-370.
5.Dunaevsky, Y. E., Gladysheva, I. P., Pavlukova, E.
B., Beliakova, G. A., Gladyshev, D. P., Papisova, A. I., Larionova, N.
I., and Belozersky, M. A. (1997) Physiol. Plantarum, 101,
483-488.
6.Dunaevsky, Y. E., Pavlukova, E. B., and Belozersky,
M. A. (1996) Biochem. Mol. Biol. Int., 40,
199-208.
7.Erlanger, B. F., Kokowsky, N., and Cohen, W. (1961)
Arch. Biochem. Biophys., 95, 271-278.
8.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) J. Biol. Chem., 193, 265-275.
9.Davis, B. J. (1964) Ann. N. Y. Acad. Sci.,
121, 404-427.
10.Freedman, M. H., Grossberg, A. L., and Pressman,
D. (1968) Biochemistry, 7, 1941-1950.
11.Smith, E. L. (1977) in Methods in
Enzymology (Hirs, C. W., and Timasheff, S. N., eds.) Academic
Press, N. Y.-San Francisco-London, pp. 156-161.
12.Thomsen, J., and Bayne, S. J. (1988) J. Prot.
Chem., 7, 295-296.
13.Dean, P. D., Johnson, W. S., and Middle, F. A.
(1985) Affinity Chromatography. Methods, IRL Press,
Oxford-Washington, DC.
14.Chigi, H., Tanaka, S., and Isawa, M. (1980)
Agric. Biol. Chem., 44, 205-207.
15.Kaur-Sawhney, R., Shin, L., Cegielska, T., and
Galston, A. W. (1982) FEBS Lett., 145, 345-349.
16.Graham, J. S., Pearse, G., Merryweather, J.,
Titani, K., Ericsson, L. H., and Ryan, C. A. (1985) J. Biol.
Chem., 260, 6561-6564.
17.Katayama, H., Soezima, Y., Fujimura, S., Terada,
S., and Kimoto, E. (1994) Biosci. Biotech. Biochem., 58,
2004-2008.
18.Tamir, S., Bell, J., Finlay, T. H., Sakal, E.,
Smirnoff, P., Gaur, S., and Birk, Y. (1996) J. Protein
Chem., 15, 219-229.
19.Yoshikawa, M., Yokota, K., and Hiraki, K. (1985)
Agric. Biol. Chem., 49, 367-371.
20.Kiyohara, T., and Iwasaki, T. (1985) Agric.
Biol. Chem., 49, 581-588.
21.Ikeda, K., and Kusano, T. (1983) Agric. Biol.
Chem., 47, 481-486.
22.Belozersky, M. A., Dunaevsky, Y. E., Musolyamov,
A. X., and Egorov, T. A. (1995) FEBS Lett., 371,
264-266.
23.Belozersky, M. A., Dunaevsky, Y. E., Musolyamov,
A. X., and Egorov, T. A. (2000) IUBMB Life, 49,
273-276.
24.Valdés-Rodríguez, S., Segura-Nieto,
M., Chagolla-López, A., Verver y Vardas-Cortina, A.,
Martínez-Gallardo, N., and Blanco-Labra, A. (1993) Plant
Physiol., 103, 1407-1412.
25.Richardson, M., and Cossins, L. (1974) FEBS
Lett., 45, 11-13.
26.Park, S. S., Abe, K., Kimura, M., Urisu, A., and
Yamasaki, N. (1997) FEBS Lett., 400, 103-107.