Isolation, Crystallization, and Investigation of Ribosomal Protein S8 Complexed with Specific Fragments of rRNA of Bacterial or Archaeal Origin
S. V. Tishchenko1*, J. M. Vassilieva1, O. B. Platonova1, A. A. Serganov1, N. P. Fomenkova1, E. S. Mudrik1, W. Piendl2, Ch. Ehresmann3, B. Ehresmann3, and M. B. Garber1
1Institute of Protein Research, Russian Academy of Sciences, Pushchino, Moscow Region, 142290 Russia; fax: (095) 924-0493; E-mail: sveta@vega.protres.ru2Institute of Medical Chemistry and Biochemistry, University of Innsbruck, Austria; fax: ++43-512-507-2872; E-mail: Wolfgang.Piendl@uibk.ac.at
3Istitut de Biologie Moleculaire et Cellulaire du CNRS, Strasbourg Cedex, France; fax: 33-(0)3-88-60-22-18; E-mail: Chantal.Ehresmann@ibmc.u-strasbrg.fr
* To whom correspondence should be addressed.
Received May 14, 2001
The core ribosomal protein S8 binds to the central domain of 16S rRNA independently of other ribosomal proteins and is required for assembling the 30S subunit. It has been shown with E. coli ribosomes that a short rRNA fragment restricted by nucleotides 588-602 and 636-651 is sufficient for strong and specific protein S8 binding. In this work, we studied the complexes formed by ribosomal protein S8 from Thermus thermophilus and Methanococcus jannaschii with short rRNA fragments isolated from the same organisms. The dissociation constants of the complexes of protein S8 with rRNA fragments were determined. Based on the results of binding experiments, rRNA fragments of different length were designed and synthesized in preparative amounts in vitro using T7 RNA-polymerase. Stable S8-RNA complexes were crystallized. Crystals were obtained both for homologous bacterial and archaeal complexes and for hybrid complexes of archaeal protein with bacterial rRNA. Crystals of the complex of protein S8 from M. jannaschii with the 37-nucleotide rRNA fragment from the same organism suitable for X-ray analysis were obtained.
KEY WORDS: ribosomal protein S8, rRNA-protein complex, dissociation constant, Thermus thermophilus, Methanococcus jannaschii
Study of the nature of protein-rRNA complexes is a topical problem of modern molecular biology. Structural studies of rRNA-protein complexes are the most direct and precise method of analysis of these interactions. Because ribosomal proteins are most conservative during evolution, their complexes with specific RNA fragments provide an interesting model for studying RNA-protein interactions.
Ribosomal protein S8 from E. coli plays a key role in assembling the small ribosomal subunit [1, 2] and regulating the expression of the genes encoding ribosomal proteins of the spc operon [3]. During assembly of the 30S subunit, protein S8 independently of other ribosomal proteins binds with the central domain of 16S rRNA and together with proteins S15, S6, S18, and S11 forms the side projection of the 30S subunit, the “platform”.
The major region of protein S8 binding on 16S rRNA was determined by partial hydrolysis with restriction endonucleases [4] and UV-cross-linking [5]. It is located in helix 21 of 16S rRNA and consists of a double RNA strand with a conserved central region where the A-form of rRNA is significantly deformed. The binding site consists of nucleotides 588-602 and 633-651 of 16S rRNA [6]. Using site-directed mutagenesis [6], in vitro Selex experiments [7], and NMR spectroscopy [8, 9], it was shown that the conserved central region containing three protruding bases (A595, U641, and A642) plays a key role in the specific binding of protein S8. Using the hydroxyl radical “footprint” procedure, it was shown that protein S8 has additional contacts in the region of helix 25 of 16S rRNA [10].
The binding sites of protein S8 on 16S rRNA are similar in E. coli and T. thermophilus. It was shown that the binding affinity of protein S8 from T. thermophilus for the specific fragment of 16S rRNA of E. coli is similar to that characteristic of its binding with its own 16S rRNA fragment [11].
When protein S8 is synthesized in excess with respect to 16S rRNA, it acts as a repressor of translation of the genes encoding the ribosomal proteins located in the spc operon. The mRNA fragment that is recognized and protected by protein S8 was isolated and characterized by treating RNA-protein complexes with restriction endonucleases [12, 13]. It was found that, although the structures of mRNA and rRNA binding sites for protein S8 are very similar, the apparent dissociation constant (Kd) for the complex of protein S8 with the specific mRNA fragment is fivefold higher than that of the complex with rRNA.
The structures of protein S8 from two eubacteria (Bacillus stearothermophilus and T. thermophilus) are now known [14, 15]. The structure of the smallest fragment of E. coli rRNA that binds protein S8 (free and protein S8-bound) was determined by NMR [8, 9]. Recently, the crystal structure of the small ribosomal subunit from T. thermophilus, where protein S8 is surrounded by its native microenvironment, has been determined to 3 Å resolution [16].
Determination of spatial structures of binary S8-RNA complexes from different organisms with high resolution would allow comparative analysis of RNA-protein interactions in these complexes and reveal the general principles that determine the specificity and stability of these complexes.
The goal of this work was to study RNA-binding properties of the ribosomal protein S8 from T. thermophilus and. M. jannaschii, to determine the minimal specific fragment of 16S rRNA that forms a stable complex with protein S8, and to obtain crystals of S8-RNA complex suitable for X-ray analysis.
MATERIALS AND METHODS
Isolation of 16S rRNA fragments and protein S8 from T. thermophilus and M. jannaschii. The gene encoding a 41-nucleotide fragment of 16S rRNA from T. thermophilus was cloned in the pUT7 vector derived from the pUC119 plasmid [17]. The other rRNA fragments of different length were obtained using artificial DNA templates that, in addition to besides the rRNA-complementary sequence, contained the sequence of one strand of T7 RNA polymerase primer. DNA template was annealed with the other primer strand at 90°C for 3 min. The rRNA fragments were synthesized using in vitro transcription mediated by T7 RNA polymerase [18]. The 41-nucleotide rRNA fragment from T. thermophilus was isolated using chromatography (gel filtration) and preparative electrophoresis under denaturing conditions. The samples were gel filtered on Sephadex G-75 in buffer containing 50 mM sodium acetate, pH 5.5, and 2 mM MgCl2. The other RNA fragments were isolated by preparative denaturing electrophoresis of nucleic acids in 16% polyacrylamide gel using TBE buffer containing 100 mM Tris-borate, pH 8.2, and 2 mM EDTA, in 8 M urea [19]. The band corresponding to the desired RNA fragment was excised from the gel, and the gel fragment was ground, placed in the buffer containing 50 mM Tris-HCl (pH 7.5) and 1 mM EDTA, and incubated with shaking for 16-17 h at room temperature. The rRNA-containing solution was then filtered through a 0.22-µm filter. Final purification of the RNA fragments was performed using anion-exchange chromatography on DEAE-Toyopearl. The rRNA solution was applied to a column equilibrated with 50 mM Tris-HCl, pH 7.5, and eluted with a NaCl gradient (0-1 M NaCl) prepared in 50 mM Tris-HCl, pH 7.5. The RNA-containing fractions were precipitated by ethanol.
Isolation of proteins TthS8 and MjaS8. The proteins were isolated from overexpressing E. coli strains. Protein TthS8 was isolated from the strain BL21(DE3)/pET3-tthS8 as described before [11] with some modifications. The protein MjaS8 was isolated from the overexpressing strain BL21(DE3)pET-mjaS8/pUBS520 provided by Dr. W. Piendl (Austria) using the same procedure. This strain bears the pET11a vector with inserted gene of protein MjaS8 and the pUBS520 vector with inserted gene of tRNAArgAGA/AGG [20, 21]. Overexpressing E. coli strain B834(DE3) auxotrophic by methionine was used for isolation of the modified protein MjaS8 in which methionine residues are replaced by selenomethionine. The biomass was grown in minimal medium supplemented with selenomethionine [22]. All solutions used for protein isolation contained 5 mM DTT and 0.2 mM EDTA [23].
Kd values of the RNA-containing complexes were determined using a filter binding procedure [6].
Crystallization of complexes of protein S8 with specific rRNA fragments. Complex of protein TthS8 and rRNA fragment from T. thermophilus. A solution of protein TthS8 prepared in 50 mM sodium cacodylate buffer (pH 6.5) containing 200 mM KCl, 100 mM MgCl2, and 1 mM DTT was concentrated to 20 mg/ml on a Centricon with 10-kD pores (Amicon, USA). A concentrated solution of protein S8 was then mixed in equimolar ratio with the rRNA solution at the same concentration prepared in 20 mM sodium cacodylate buffer (pH 6.5) containing 10 mM MgCl2, and the sample was heated at 48°C for 10 min. The mixture was allowed to react at 4°C for 1 h to form the S8-RNA complex. Then the solution was mixed with 100 mM sodium cacodylate buffer, pH 6.5, containing 8% polyethylene glycol monomethyl ester 5K at 4 : 1 ratio and equilibrated against this solution by the method of vapor diffusion in a hanging drop at 4°C. Two days later, crystals of bipyramidal shape appeared; their sizes in one week reached 120 × 50 × 50 µm.
Complexes of protein MjaS8 with rRNA fragments from T. thermophilus. Protein MjaS8 was transferred by dialysis into 50 mM sodium cacodylate buffer (pH 6.5) containing 40 mM NaCl and then concentrated to 36 mg/ml. Complexes of protein MjaS8 with 41- and 37-nucleotide fragments of rRNA were obtained as described above for the homologous TthS8 complex. Ammonium sulfate was used as a precipitant. A hanging drop containing 2 µl of S8-RNA complex and 1 µl of 2.8 M ammonium sulfate buffered with 100 mM sodium cacodylate (pH 6.5) was equilibrated against 100 mM sodium cacodylate buffer (pH 6.5) containing 2.2 M ammonium sulfate at 4°C. The crystals formed also had bipyramidal shape and reached sizes of 800 × 400 × 400 µm.
Complex of protein MjaS8 with rRNA fragment from M. jannaschii was crystallized under the same conditions as the hybrid complex.
RESULTS AND DISCUSSION
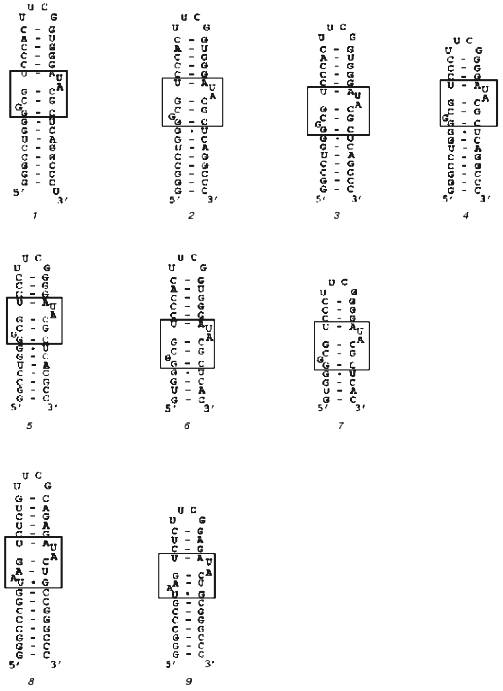
Our goal was to synthesize an rRNA fragment whose complex with protein S8 would yield crystals suitable for X-ray analysis. We synthesized and purified nine different specific fragments of 16S rRNA from T. thermophilus and M. jannaschii (Fig. 1). It is known that homologous ribosomal proteins from different organisms form hybrid complexes with cognate rRNA [24, 25]. In many cases, ribosomes containing cognate proteins can function normally. For this reason, for crystallization we used both homologous and hybrid S8-RNA complexes. The Kd values of the homologous and hybrid complexes formed by these rRNA fragments with protein S8 from these organisms were determined using filter-binding assay (Table 1).
Table 1. Apparent dissociation constants (Kd) of complexes of protein S8 from T. thermophilus and M. jannaschii with 16S rRNA fragments from T. thermophilus (Tth) of different length and an rRNA fragment from M. jannaschii (Mja)Fig. 1. Secondary structures of rRNA fragments from T. thermophilus (1-7) with length of 42 (1), 41 (2), 39 (3), 37 (4), 35 (5), 33 (6), and 29 (7) nucleotides and M. jannaschii (8, 9) with the length of 41 (8) and 37 (9) nucleotides. The regions that determine protein S8 binding are shown in the frames.
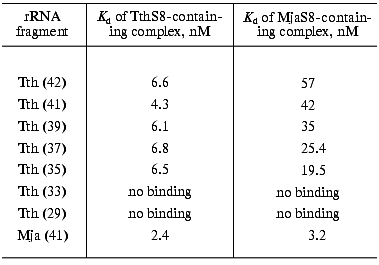
Note: Here and in Table 2 the length of RNA fragments (in nucleotides) is shown in parentheses.
We discovered that the Kd of the hybrid complex of ribosomal protein MjaS8 with the specific rRNA fragments from T. thermophilus is significantly higher than that of the homologous S8-RNA complexes from T. thermophilus, M. jannaschii, and hybrid complex of protein TthS8 with the specific rRNA from M. jannaschii. These results were confirmed when studying competition of rRNA fragments (from T. thermophilus and M. jannaschii) for binding with protein S8 isolated from these organisms (data not shown). We also found that Kd values of complexes between specific rRNA fragments from T. thermophilus and protein MjaS8 depend on the length of these fragments: the shorter the fragment, the lower the Kd value (Table 1). We showed that protein TthS8binds with rRNA fragments from T. thermophilus and M. jannaschii with virtually equal affinity, whereas protein MjaS8binds with rRNA fragments from T. thermophilus with much lower (five- to tenfold) affinity than the homologous fragments from M. jannaschii. Apparently, protein MjaS8 exhibits higher selectivity in binding specific rRNA structures than protein TthS8. It is known that the amino acid sequence of archaeal ribosomal proteins is more similar to that of eukaryotic rather than prokaryotic homologs [26]. Only ~30% of amino acid residues in protein MjaS8 are identical to those of bacterial proteins S8, whereas ~50% of amino acids residues of MjaS8 are identical to those of eukaryotic homologs. So far, archaeal protein S8 has not been studied as intensively as its bacterial homolog.
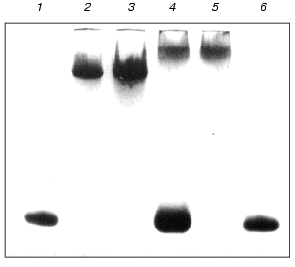
We also studied the effect of Mg2+ on the formation of complexes of proteins MjaS8 and TthS8 with the 41-nucleotide rRNA fragment from T. thermophilus. Using PAGE under nondenaturing conditions, it was shown that the absence of Mg2+ in the reaction medium does not affect the formation of the complex between MjaS8 and this rRNA fragment, but attenuates the interaction of TthS8 with rRNA (Fig. 2). This finding is indicative of different nature of the interaction of proteins TthS8 and MjaS8 with the rRNA fragment from T. thermophilus. It is likely that, during interaction between MjaS8 and rRNA, Mg2+ does not participate in formation or stabilization of the secondary and tertiary structures of the protein-binding site in rRNA and/or is not located on the contact surface between the protein and rRNA in the complex. The presence of Mg2+ is apparently required for production of a stable complex between rRNA and protein TthS8. It was shown earlier that an rRNA fragment from E. coli that specifically binds with protein TthS8 contains a Mg2+-binding site that participates in stabilization of the secondary and ternary RNA structures [9]. Thus, rRNA from the hyperthermophilic archaea M. jannaschii (growth temperature 85°C) does not require the presence of Mg2+ for retaining its structural stability, whereas rRNA from T. thermophilus (growth temperature 75°C) forms complex with protein S8 only in the presence of Mg2+, and the structure of rRNA from E. coli is completely melted in the absence of Mg2+ [26].
To minimize the rRNA fragment, we successively removed nucleotide pairs from the upper and lower parts of the rRNA strand, thereby obtaining the minimal rRNA fragment that retained the ability to bind proteins MjaS8 and TthS8. Further decrease in the length of the rRNA fragment in its lower part caused complete loss of its ability to bind protein S8. This indicates that, besides the binding site with protruding bases, the rRNA molecule contains another binding site for protein S8 in the lower part of the strand. This is consistent with the data on the presence of a nonspecific binding site in this region of RNA from E. coli [27]. The minimal 37-nucleotide fragment of rRNA from M. jannaschii was obtained in a similar way.Fig. 2. Electrophoresis of S8-RNA complexes under nondenaturing conditions (8% polyacrylamide gel, 0.09 M Tris-borate buffer, pH 8.3, 1 mM MgCl2): 1, 6) control RNA; 2) complex MjaS8-rRNA formed in the absence of Mg2+; 3) complex MjaS8-rRNA formed in the presence of 5 mM Mg2+; 4) complex TthS8-rRNA formed in the absence of Mg2+; 5) complex TthS8-rRNA formed in the presence of 5 mM Mg2+.
For crystallization we used S8-RNA complexes of different composition (Table 2). The first crystallized complex was that formed by protein TthS8 and the 41-nucleotide fragment of rRNA from T. thermophilus. The crystals reflected X-rays to 8-Å resolution, which is insufficient for determination of the spatial structure of the molecule. For this reason, we continued searching for a complex optimal for structural studies. Next, we obtained crystals of hybrid complexes of protein MjaS8 with the fragments of 16S rRNA from T. thermophilus. The crystals of the hybrid complex with the 41-nucleotide rRNA fragment reflected X-rays to 3.6-Å resolution, which is better but still insufficient for determination of the structure of the complex. Crystals of the second hybrid complex of MjaS8 with the 37-nucleotide fragment of rRNA from T. thermophilus reflected X-rays to virtually the same resolution (Table 2). We also showed that the RNA preparation obtained by preparative electrophoresis is purer than that obtained by the chromatographic procedure, and the quality of the crystals containing the purer rRNA preparation is higher.
Table 2. Characteristics of crystals of
S8-RNA complexes of different composition
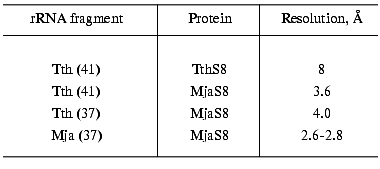
We could not crystallize the complexes of protein MjaS8 with the 42-, 39-, and 35-nucleotide fragments of rRNA from T. thermophilus and with the 41-nucleotide fragment of rRNA from M. jannaschii.
The best results were obtained when crystallizing the homologous S8-RNA complex from M. jannaschii containing the 37-nucleotide rRNA fragment from M. jannaschii. The crystals reflected X-rays to 2.8-Å resolution, which allowed us to study the structure of this complex. We substituted the methionine residues in the protein with selenomethionine and then crystallized the complex of rRNA fragment with the modified protein MjaS8. As a result, the quality of the crystals was improved and the resolution increased to 2.6 Å. Diffraction analysis of these crystals was performed on synchrotrons in Hamburg (Germany, EMBL XII DESY) and Grenoble (France, BM30 ESRF) at 100 K using cryosolution containing 0.1 M sodium cacodylate buffer, pH 6.5, 30% glucose, and 2.5 M ammonium sulfate. An electron-dense map with 2.6-Å resolution was obtained.
This study was supported by the Russian Academy of Sciences and the Russian Foundation for Basic Research (project No. 00-04-48208). The work of W. Piendl was supported by the Austrian Science Foundation (grant P14550-GEN) and the Russian-Austrian Collaboration Program (grant No. 1.5). The work of M. Garber was supported by a grant from HHMI.
REFERENCES
1.Held, W. A., Ballou, B., Mizushima, A., and Nomura,
M. (1974) J. Biol. Chem., 249, 3103-3111.
2.Nowtony, V., and Nierhaus, K. H. (1988)
Biochemistry, 27, 7051-7055.
3.Dean, D., Yetes, J. L., and Nomura, V. (1981)
Nature, 289, 89-91.
4.Ungewickell, E., Garret, R. A., Ehresmann, Ch.,
Stiegler, P., and Fellner, P. (1975) Eur. J. Biochem.,
51, 165-180.
5.Wower, I., and Brimacombe, R. (1983) Nucleic
Acids Res.,11, 1419-1437.
6.Mougel, M., Allmang, C., Eyermann, F., Cachia, C.,
Ehresmann, B., and Ehresmann, C. (1993) Eur. J. Biochem.,
215, 787-792.
7.Monie, H., Cachia, C., Westhof, E., Ehresmann, B.,
and Ehresmann, C. (1997) RNA, 3, 255-268.
8.Kalurachchi, K., Uma, K., Zimmermann, R. A., and
Nikonowicz, E. P. (1997) Proc. Natl. Acad. Sci. USA, 94,
2139-2144.
9.Kalurachchi, K., and Nikonowicz, E. P. (1998) J.
Mol. Biol., 280, 639-654.
10.Lancaster, L., Culver, G. M., Yusupova, G. Zh.,
Cate, J. H., Yusupov, M. M., and Noller, H. F. (2000) RNA,
6, 717-729.
11.Vysotskaya, V., Tishchenko, S., Garber, M., Kern,
D., Mougel, M., Ehresmann, C., and Ehresmann, B. (1994) Eur. J.
Biochem., 223, 437-445.
12.Gregory, R. J., Cahill, P. B. F., Thurlow, D. L.,
and Zimmermann, R. A. (1988) J. Mol. Biol., 204,
295-307.
13.Ceretti, D. P., Mattheakis, L. C., Kearney, K.
R., Vu, L., and Nomura, M. (1988) J. Mol. Biol., 204,
309-329.
14.Davis, C., Ramakrishnan, V., and White, S. W.
(1996) Structure, 4, 1093-1104.
15.Nevskaya, N., Tishchenko, S., Nikulin, A.,
Al-Karadaghi, S., Liljas, A., Ehresmann, B., Ehresmann, C., Garber, M.,
and Nikonov, S. (1998) J. Mol. Biol., 279, 233-244.
16.Wimberly, B. T., Brodersen, D. E., Clemons, W.
M., Jr., Morgan-Warren, R. J., Carter, A. P., Vonrhein, C., Hartsch,
T., and Ramakrishnan, V. (2000) Nature, 407, 327-339.
17.Viera, J., and Messing, J. (1987) Meth.
Enzymol., 153, 3-11.
18.Milligan, J. F., and Unlenbeck, O. C. (1989)
Meth. Enzymol., 180, 51-62.
19.Wyatt, J. R., Chastain, M., and Puglishi, J. D.
(1991) BioTechniques, 11, 764-769.
20.Calderone, T. L., Stevens, R. D., and Oas, T. G.
(1996) J. Mol. Biol., 262, 407-412.
21.Brinkmann, U., Mattes, R. E., and Buckel, P.
(1989) Gene, 85, 109-114.
22.Leahy, D. S., Hendrickson, W. A., Aukhil, I., and
Erickson, H. P. (1992) Science, 258, 987-991.
23.Doublie, S. (1997) Meth. Enzymol.,
267, 523-529.
24.Gourse, R., Thurlow, D., Gerbi, S., and
Zimmermann, R. (1981) Proc. Natl. Acad. Sci. USA, 78,
2722-2726.
25.Zimmermann, R., Thurlow, D., Finn, R., Marsh, T.,
and Ferrett, L. (1980) in RNA Polymerase, tRNA and Ribosomes: Their
Genetics and Evolution (Osawa, S., Ozeki, H., Uchida, H., and Yura,
T., eds.) University of Tokyo Press, Tokyo, pp. 569-584.
26.Ramirez, C., Koepke, A. K. E., Yang, D.-C.,
Boeckh, T., and Matheson, A. T. (1993) in The Biochemistry of
Archaea (Archaebacteria) (Kates, M., Kushner, D., and Higgins, S.
J., eds.) Elsevier, Amsterdam, pp. 439-466.
27.Mougel, M., Eyermann, F., Westhof, E., Romby, P.,
Expert-Bezancon, A., Ebel, J.-P., Ehresmann, B., and Ehresmann, C.
(1987) J. Mol. Biol., 198, 91-107.