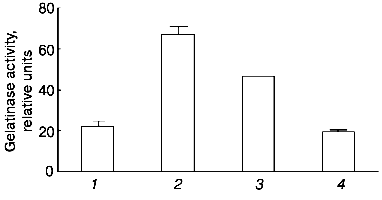Plasmin-Independent Gelatinase B (Matrix Metalloproteinase-9) Release by Monocytes under the Influence of Urokinase
M. Yu. Menshikov1*, E. P. Elizarova1, E. Kudryashova1, A. V. Timofeyeva1, Yu. Khaspekov1, R. Sh. Beabealashvilly1, and A. Bobik2
1Institute of Experimental Cardiology, Cardiology Research Center, 3-ya Cherepkovskaya ul. 15a, Moscow, 121552 Russia; fax: (095) 414-6719; E-mail: myumensh@cardio.ru2Baker Medical Institute, Victoria 3181, Australia; E-mail: abobik@baker.edu.au
* To whom correspondence should be addressed.
Received June 4, 2001
It is shown that the release of matrix metalloproteinase-9 (gelatinase B) by THP-1 and U937 cells into conditioned media is increased under the action of recombinant single-chain urokinase. This effect is not accompanied by proteolytic activation of gelatinase B and is related to release of a pro-form of the enzyme. The action of urokinase on monocytes is time-dependent and becomes significant 12-24 h after the beginning of cell incubation. The dependence of the effect on the concentration of urokinase is characterized by half-maximum at about 20 nM and saturation at about 200 nM. The urokinase-induced gelatinase B release is not dependent on the action of plasmin because plasmin inhibitors aprotinin and alpha2-antiplasmin do not abolish this action. Additionally, tissue type plasminogen activator does not induce gelatinase B release by monocytes as observed under the action of urokinase. Nevertheless, the catalytic activity of urokinase participates in the development of the observed effect because it is significantly depressed by the natural urokinase inhibitor PAI-1. The effect of urokinase is completely abolished by actinomycin D and cycloheximide, indicating the participation of transcription and translation processes in its development.
KEY WORDS: urokinase, plasmin, tissue-type plasminogen activator, matrix metalloproteinases, gelatinase B, monocytes
Abbreviations: MMP) matrix metalloproteinase; PAI-1) plasminogen activator inhibitor type-1; PMSF) phenylmethylsulfonyl fluoride; uPA) urokinase plasminogen activator; tPA) tissue type plasminogen activator; TIMP) tissue inhibitor of matrix metalloproteinases; LPS) lipopolysaccharide.
Proteolytic degradation of extracellular matrix is an important factor
in tissue remodeling in angiogenesis, embryogenesis, wound healing,
scar formation, fibrosis, cell invasion, tumor growth, and many other
physiological and pathological processes [1]. The
major factor of extracellular matrix proteolysis is the activity of
matrix metalloproteinases (MMPs), which belong to a family of
Zn-dependent neutral proteolytic enzymes expressed in different types
of cells [2-5].
In leukocytes (neutrophils, macrophages, lymphocytes, eosinophils) a significant part of the metalloproteinase activity is represented by gelatinase B (MMP-9; 92-kD MMP) [6-9] having substrate specificity similar to that of gelatinase A (MMP-2; 72-kD MMP) and degrading type IV collagen, the structural component of basal membrane.
The expression of gelatinase B in these cells can be induced by interleukin-1beta, tumor necrosis factor-alpha, transforming factor-beta, lymphotoxin, LPS [10-12], and by other agents stimulating cell migration. The expression and release of gelatinase B is frequently accompanied by expression of another proteolytic enzyme, urokinase.
Urokinase, as well as tissue-type plasminogen activator, participates in fibrinolysis by inducing the formation of plasmin, a serine proteinase disrupting fibrin clots. Moreover, urokinase complexed with its membrane receptor, a glycosyl phosphatidylinositol-anchored membrane protein is also involved in stimulation of migration of different cell types [13, 14] and also activates growth factors [15, 16], increasing in this way the invasiveness of different cell types.
The expression of urokinase is controlled by the same agents (growth factors, interleukins) and occurs under physiological and pathological conditions similar to those in which the expression of gelatinase B occurs [17-19]. Being an agent affecting cell migration and cell invasion processes, urokinase could induce the expression and release of proteins participating in these processes. This might suggest that urokinase induces the expression of matrix metalloproteinases, which are immediate participants of cell invasion processes.
The purposes of the present study were the following: to explore the action of urokinase on the release of matrix metalloproteinases by monocytes; to identify the spectrum of the enzymes released; to evaluate the participation of plasmin in the development of the urokinase effect.
MATERIALS AND METHODS
Chemicals. The following chemicals were used in the present study: tissue-type plasminogen activator (tPA) (Boehringer Ingelheim, Germany); actinomycin D, cycloheximide, alpha2-antiplasmin, phenylmethylsulfonyl fluoride (PMSF), and leupeptin (Sigma, USA); plasminogen activator inhibitor type-1 (PAI-1) (American Diagnostica, USA); aprotinin (Bayer, Germany); culture medium RPMI 1640 and fetal calf serum (Life Technologies, Inc., USA). Recombinant single-chain urokinase was expressed in Escherichia coli and purified as described previously [20]. Other chemicals were of the best quality available.
Cell culture and cell stimulation. Monocytes U937 and THP-1 were cultured at 37°C in RPMI 1640 medium containing penicillin/streptomycin and 10% fetal calf serum (FCS), and the medium was replaced every 3 days. Before each experiment, the concentration of cells was adjusted to 2*106 per ml using RPMI 1640 containing 0.5% FCS.
Cell number and cell viability were evaluated in a Goryaev chamber [21] by using Trypan Blue exclusion test.
Stimulation of cells by urokinase or by tissue-type plasminogen activator (tPA) was performed in 96-well plates (Costar, USA) in a 200-µl volume of cell suspension. If not otherwise stated, the duration of stimulation was 48 h with urokinase concentration 20 nM. After centrifugation of cells (12,000 rpm, table-top centrifuge), the supernatants (conditioned media) were collected and kept frozen until use for testing gelatinase activity by zymography.
The inhibitors of plasmin (alpha2-antiplasmin, aprotinin) as well as actinomycin D and cycloheximide, where indicated, were added to cell suspension 1 h before urokinase.
To evaluate the influence of inhibitor of plasminogen activator type 1 (PAI-1) on cell stimulation by urokinase, a mixture containing 400 nM of urokinase and 800 nM of PAI-1 in RPMI 1640 medium (25 µl) was incubated for 1 h at room temperature. Following incubation, the mixture was added to the cell suspension in a volume giving a final urokinase concentration of 20 nM.
Zymography. The release of matrix metalloproteinases by monocytes was estimated by zymography. Samples of cell-conditioned medium (10-20 µl) were mixed with sample buffer (0.0625 M Tris-HCl, pH 6.8, 2% SDS, 10% glycerol (v/v), 0.001% bromophenol blue) containing no beta-mercaptoethanol and subjected to SDS-PAGE in 7.5% polyacrylamide gel containing 0.2% gelatin at 4°C and 150 V [22]. Molecular masses of proteins separated were determined using the 10-250 kD protein standards (Bio Rad, Germany). After running, the gels were washed in 2.5% Triton X-100 for 1.5 h followed by 18-h incubation of the gels in buffer containing 50 mM Tris-HCl (pH 8.0), 50 mM NaCl, 0.05% Brij 35, and 10 mM CaCl2. Where indicated, the incubation buffer contained 10 µg/ml aprotinin, 2 mM PMSF, 10 µg/ml leupeptin, or 10 mM EDTA (“no Ca2+”) instead of CaCl2. After incubation, the gels were stained by 0.25% Coomassie Brilliant Blue G-250 in 40% (v/v) methanol and 10% (v/v) acetic acid followed by washout of excessive dye by a mixture of 40% (v/v) methanol and 10% (v/v) acetic acid. Localization and expression of proteolytic activity was determined by evaluating the intensity of unstained bands on the gelatin background. Zymograms were subjected to densitometric analysis using PCBAS software. Gelatinolytic activity was expressed in arbitrary units.
RESULTS
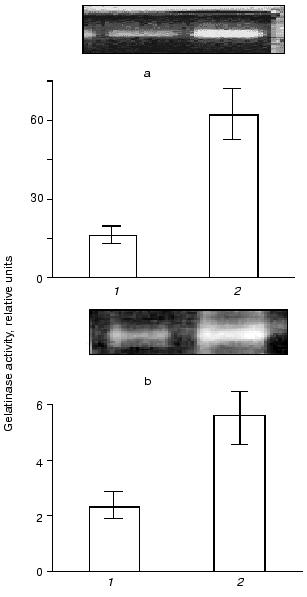
As shown in Fig. 1, THP-1 and U937 monocytes growing in culture release proteolytic enzyme having a molecular mass of about 90 kD that can be revealed by gelatin zymography. Incubation of monocytes in the presence of recombinant wild-type urokinase increases gelatinase release by the cells. This effect is of similar value in THP-1 and in U937 cell lines.
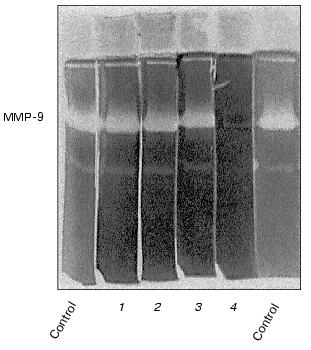
The enzyme released by monocytes is not sensitive to aprotinin, leupeptin, and PMSF, but it is inhibited by calcium withdrawal in the presence of EDTA (Fig. 2) and can be identified as a matrix metalloproteinase with molecular mass 92 kD, corresponding to matrix metalloproteinase-9 (MMP-9), or gelatinase B [23].
Fig. 1. Effect of urokinase type plasminogen activator on gelatinase release by THP-1 and U937 monocytes. THP-1 (a) or U937 (b) cells were incubated for 48 h with saline (control) (1) and in the presence of 20 nM urokinase plasminogen activator (uPA) (2). After the incubation, conditioned medium was collected and subjected to zymography as described in “Materials and Methods”. Typical zymograms and densitometric data of three independent experiments are presented.
In both cell types, the development of the effect of urokinase is not accompanied by the appearance of additional bands of gelatinase activity with molecular masses less than 92 kD (Fig. 1); this indicates the absence of proteolytic activation of gelatinase B during its release by the cells.Fig. 2. Evaluation of sensitivity of gelatinase to proteinase inhibitors and Ca2+ abolition. Polyacrylamide gels following separation of conditioned medium were incubated in the presence of 10 µg/ml aprotinin (1), 2 mM PMSF (2), 10 µg/ml leupeptin (3), or with 10 mM EDTA (4) instead of CaCl2.
Numeric effects of urokinase on different cell types are related to its active site function as well as to formation of a complex between urokinase and its membrane receptor, a glycosyl phosphatidylinositol-anchored protein [13]. Plasminogen activator inhibitor of type 1 (PAI-1) is one of the natural inhibitors of urokinase that forms a covalently bound and proteolytically inactive complex with this enzyme [24, 25].
To evaluate the participation of the active site in the development of the observed effect, we preincubated urokinase in the presence of 2-fold excess of PAI-1 under conditions leading to the formation of a complex between urokinase and its inhibitor [24]. Incubation of urokinase in the presence of PAI-1 results in a significant (but not complete) decrease in the ability of urokinase to induce the release of gelatinase B by THP-1 cells (Fig. 3). It can be concluded, therefore, that the formation of complex between urokinase and its inhibitor significantly (but not completely) suppresses the effect of the MMP release.
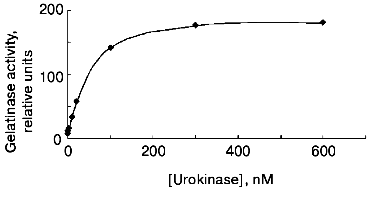
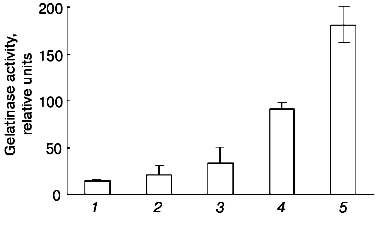
The data of Fig. 4 show that if the cells are incubated for 48 h at 37°C, the concentration dependence of the effect of urokinase is characterized by its amplification in the 3 to 100 nM range, following by its saturation at higher dosage. The half-maximum effect of urokinase is observed at concentrations close to 20 nM.Fig. 3. Decrease in the urokinase effect on gelatinase B release by THP-1 cells in the presence of plasminogen activator inhibitor type 1. Gelatinase B activity was estimated by zymography in conditioned medium of THP-1 cells incubated in the absence of urokinase (control) (1) and in the presence of: 20 nM urokinase (2), 20 nM urokinase and 40 nM PAI-1 (3), 40 nM PAI-1 (4). Data of three independent experiments are presented.
The main target of proteolytic action of urokinase is plasminogen, which is activated by transformation into plasmin-disrupting fibrin clots and participates in a number cellular effects of urokinase [26]. Besides urokinase, tissue-type plasminogen activator (tPA), another fibrinolytic agent used in clinical practice, has the ability to transform plasminogen into plasmin. At the same time, as shown in Fig. 5, tPA fails to induce gelatinase B release by THP-1 cells at concentrations 20 and 200 nM, where the effect of urokinase develops. These data suggest that plasmin is not a mediator of this effect.Fig. 4. Concentration dependence of the effect of urokinase on gelatinase B release by THP-1 cells. The cells were incubated for 48 h in the presence of 0 (saline control), 0.3, 1, 3, 10, 20, 100, 200, and 1000 nM urokinase following by analysis of the conditioned medium by zymography.
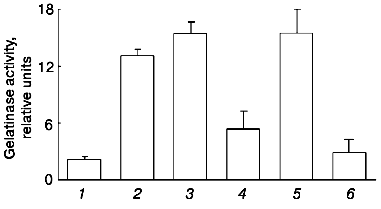
To clearly resolve the question of the participation of plasmin in gelatinase B release by monocytes under the action of urokinase, we evaluated the possibility for plasmin inhibitors, alpha2-antiplasmin and aprotinin, to affect the action of urokinase at concentrations sufficient for complete suppression of plasmin activity [27, 28]. These experiments showed that neither alpha2-antiplasmin nor aprotinin significantly affects gelatinase B release under the effect of urokinase (Fig. 6). Similar results were obtained with U937 cells (data not shown). Thus, we concluded that the ability of urokinase to induce gelatinase B release by monocytes is not mediated by activation of plasminogen.Fig. 5. Comparative study of the effects of urokinase (uPA) and tissue type (tPA) plasminogen activators on the release of gelatinase B by THP-1 cells.
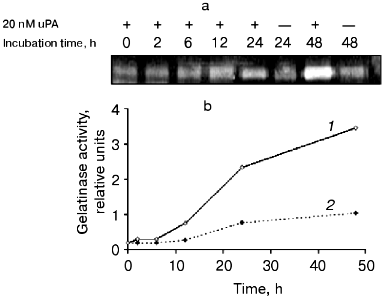
The dependence of gelatinase B release on the time of incubation of THP-1 cells with urokinase (Fig. 7) is characterized by a significant effect after 12 h of incubation with subsequent increase in the enzyme amount on increasing the incubation duration. Similar results were obtained when U937 cells were incubated with urokinase (data not shown). The long duration of the effect of urokinase indicates that it is not limited by secretion of matrix metalloproteinase from cells into the culture medium. As mentioned above (see Fig. 1), the increase in the gelatinase B activity in culture medium is not a result of enzyme activation. This suggests that the effect of urokinase is most probably related to processes of transcription and protein synthesis. To test this hypothesis, we studied the possibility of an RNA synthase inhibitor (actinomycin D) and protein synthesis inhibitor (cycloheximide) to influence the action of urokinase on THP-1 cells.Fig. 6. Inability of the plasmin inhibitors aprotinin and alpha2-antiplasmin to modify the urokinase-stimulated release of gelatinase activity by THP-1 cells. THP-1 cells were incubated with no additions (1) and in the presence of: 20 nM urokinase (2), urokinase and aprotinin (300 activity units per ml medium) (3), aprotinin (300 activity units per ml medium) (4), urokinase and 5 µg/ml alpha2-antiplasmin (5), 5 µg/ml alpha2-antiplasmin (6).
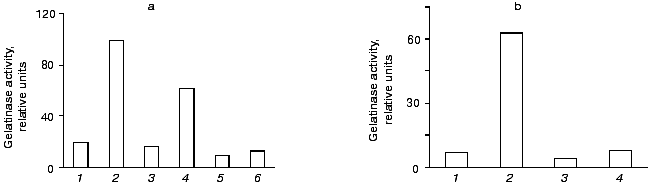
As shown in Fig. 8a, the increase in gelatinase release observed upon urokinase addition was suppressed about 2-fold in the presence of 0.1 µg/ml cycloheximide, and it was completely abolished when the inhibitor was added to 0.5 µg/ml. Actinomycin D completely inhibited this effect at concentrations as low as 0.1 µg/ml (Fig. 8b). These data indicate that the major reason for gelatinase B release by monocytes is the urokinase-induced stimulation of transcription and translation.Fig. 7. Time dependence of gelatinase release by monocytes induced by 20 nM urokinase. a) Typical zymogram of conditioned medium of THP-1 cells incubated at 37°C with 20 nM urokinase for 0, 2, 6, 12, 24, and 48 h. b) Dependence of MMP-9 activity on the time of incubation of cells in the presence (1) or absence (2) of urokinase obtained by densitometric treatment of the zymography data.
Fig. 8. Effect of cycloheximide (a) and actinomycin D (b) on urokinase-induced gelatinase release by THP-1 cells. a) THP-1 cells were incubated for 48 h either with no additions (1) or in presence of: 20 nM urokinase (2, 4, 6), 0.1 µg/ml (3, 4) or 0.5 µg/ml (5, 6) cycloheximide. b) Cells were incubated with no additions (1) or in the presence of: 20 nM urokinase (2, 4), 0.1 µg/ml actinomycin D (3, 4). The results of one of three experiments are presented.
DISCUSSION
The present study shows that urokinase-type plasminogen activator is able to induce gelatinase B release by monocyte cell lines THP-1 and U937. This effect is a part of the overall cellular action of urokinase the results in development of proliferative and migratory responses [14, 29] and might assist in strengthening the cellular invasive potential, which plays an important physiological and pathological role.
Activated urokinase is a serine proteinase that converts plasminogen into plasmin and participates in this way in fibrinolysis as well as in processes where different types of cells are involved [27, 28]. A significant factor promoting the cellular effects of urokinase is its membrane localization due to the formation of a complex between urokinase and its receptor, glycosyl phosphatidylinositol-anchored protein [13].
It is known that plasmin participates, directly or indirectly, in the proteolytic activation of some matrix metalloproteinases [30]. In particular, plasmin has been shown to activate gelatinase A in vitro [31]. Additionally, data exist suggesting that plasmin is a participant in the proteolytic cascade resulting in activation of stromelysin and, in this way, in activation of gelatinase B [32]. This activation could be assisted by an increase in the membrane-bound plasmin activity observed in THP-1 cells under the influence of some growth factors (e.g., transforming growth factor-beta) [33].
According to our data, plasmin does not participate in gelatinase B release induced by urokinase. First, tissue-type plasminogen activator does not induce an effect in monocytes similar to that of urokinase; second, alpha2-antiplasmin and aprotinin, inhibiting plasmin activity, fail to change the effect of urokinase; and third, the increase in gelatinase B release induced by urokinase is not accompanied by the appearance of the activated matrix metalloproteinase form. These data probably suggest that either the amount of cellular plasminogen is too low to participate in the activation process or that the inhibitors (alpha2-antiplasmin, TIMP-2) are present, preventing gelatinase B activation through stromelysin activity induced by exogenous urokinase. At the same time, proteolytic activity of urokinase seems to play an important role in gelatinase B release by monocytes because this effect is sensitive to PAI-1, which inhibits the proteolytic activity of urokinase.
The long duration of gelatinase B release by monocytes and its sensitivity to inhibition of transcription (actinomycin D) and translation (cycloheximide) suggest that urokinase induces activation of gelatinase B mRNA formation and protein synthesis. According to modern views, the stimulation of cells by urokinase can be mediated by signal transduction processes [34]. Another possibility for urokinase is a proteolytic activation of growth factors (transforming growth factor, basic fibroblast growth factor, hepatocyte growth factor, and some others) [35, 36] or the enhancement of secretion of these factors by cells [29], thus influencing cell growth, migration, and expression of proteins participating in these processes. The phenomenon described is probably also based on similar mechanisms.
In conclusion, our study has revealed the possibility of gelatinase B expression under the action of urokinase that is not mediated by plasmin and is not accompanied by transformation of the gelatinase into its active enzyme form; the effect is rather due to the expression of the enzyme. Further experiments using invasion models are needed to reveal the physiological importance of this effect and its significance for the regulation of invasive activity of monocytes. Also, there is clear interest in revealing extra- and intracellular mechanisms mediating this effect or promoting its development.
This study was supported by the Russian Foundation for Basic Research (grant 99-04-48641). We thank professor Dr. Vsevolod A. Tkachuk for helpful discussions during this study and the preparation of the manuscript.
REFERENCES
1.Galis, Z. S. (1999) Fibrinolysis and
Proteolysis, 13, 54-63.
2.Wilhelm, S. M., Eisen, A. Z., Teter, M., Clark, S.
D., Kronberger, A., and Goldberg, G. (1986) Proc. Natl. Acad. Sci.
USA, 83, 3756-3760.
3.Chin, J. R., Murphy, G., and Werb, Z. (1985) J.
Biol. Chem., 20, 12367-12376.
4.Murphy, G., Cockett, M. I., Ward, R. V., and
Docherty, A. J. (1991) Biochem. J., 277, 277-279.
5.Massova, I., Kotra, L. P., Fridman, R., and
Mobashery, S. (1998) FASEB J., 12, 175-195.
6.Hibbs, M. S., Hasty, K. A., Seyer, J. S., Kang, A.
H., and Mainardi, C. L. (1985) J. Biol. Chem., 260,
2493-2500.
7.Welgus, H., Campbell, E., Cury, J., Eisen, A.,
Senior, R., Wilhelm, S., and Goldberg, G. (1990) J. Clin.
Invest., 86, 1496-1502.
8.Stahle-Backdahl, M., Sudbeck, S. D., Eisen, A. Z.,
Welgus, H. G., and Parks, W. C. (1992) J. Invest. Dermatol.,
99, 497-503.
9.Weeks, B. S., Schnaper, H. W., Handy, M., Holloway,
E., and Kleinman, H. K. (1993) J. Cell. Physiol., 157,
644-649.
10.Watanabe, H., Nakanishi, I., Yamashita, K.,
Hayakawa, T., and Okada, Y. (1993) J. Cell Sci., 104,
991-999.
11.Wahl, S. M., Allen, J. B., Weeks, B. S., Wong, H.
L., and Klotman, P. E. (1993) Proc. Natl. Acad. Sci. USA,
90, 4577-4581.
12.Saarialho-Kere, U. K., Welgus, H. G., and Parks,
W. C. (1993) J. Biol. Chem., 268, 17354-17361.
13.Dano, K., Behrendt, N., Brunner, N., Ellis, V.,
Ploug, M., and Pyke, C. (1994) Fibrinolysis, 8 (Suppl.
1), 189-203.
14.Fischer, K., Lutz, V., Wilhelm, O., Schmitt, M.,
Graeff, H., Heiss, P., Nishiguchi, T., Harbeck, N., Kessler, H.,
Luther, T., Magdolen, V., and Reuning, U. (1998) FEBS Lett.,
438, 101-105.
15.Herbert, J. M., Lamarche, I., and Carmeliet, P.
(1997) J. Biol. Chem., 272, 23585-23591.
16.Mars, W. M., Zarnegar, R., and Michalopoulos, K.
(1993) Am. J. Pathol., 143, 949-958.
17.Khan, K. M. F., and Falcone, D. J. (1997) J.
Biol. Chem., 272, 8270-8275.
18.Farina, A. R., Coppa, A., Tiberio, A.,
Tacconelli, A., Turco, A., Colletta, G., Gulino, A., and Mackay, A. R.
(1998) Int. J. Cancer, 5, 721-730.
19.Rosenthal, E. L., Johnson, T. M., Allen, E. D.,
Apel, I. J., Punturieri, A., and Weiss, S. J. (1998) Cancer
Res., 58,5221-5230.
20.Stepanova, V., Bobik, A., Bibilashvili, R.,
Belogurov, A., Rybalkin, I., Domogatsky, S., Little, P. J., Goncharova,
E., and Tkachuk, V. (1997) FEBS Lett., 414, 471-474.
21.Roper, P. R., and Drewinko, B. (1976) Cancer
Res., 36, 2182-2188.
22.Laemmli, U. K. (1970) Nature, 227,
680-685.
23.Trocme, C., Gaudin, P., Berthier, S., Barro, C.,
Zaoui, P., and Morel, F. (1998) J. Biol. Chem., 273,
20677-20684.
24.Cubellis, M. V., Andreasen, P., Ragno, P., Mayer,
M., Dano, K., and Blasi, F. (1989) Proc. Natl. Acad. Sci. USA,
86, 4828-4832.
25.Ellis, V., Wun, T.-C., Behrendt, N., Ronne, E.,
and Dano, K. (1990) J. Biol. Chem., 265, 9904-9908.
26.Unkeless, J. C., Dano, K., Kellerman, G. M., and
Reich, E. (1974) J. Biol. Chem., 249, 4295-4305.
27.Lijnen, H. R., Zamarron, C., Blaber, M., Winkler,
M. E., and Collen, D. (1986) J. Biol. Chem.,
261,1253-1258.
28.Lijnen, H. R., and Collen, D. (1995)
Baillieres Clin. Haematol., 8,277-290.
29.Sitrin, R. G., Shollenberger, S. B., Strieter, R.
M., and Gyetko, M. R. (1996) J. Leukoc. Biol., 59,
302-311.
30.Dano, K., Andreasen, P. A., Groudahl-Hansen, J.,
Kristensen, P., Nielsen, L. S., and Skriver, L. (1985) Adv. Cancer
Res., 44, 139-166.
31.Mazzieri, R., Masiero, L., Zanetta, L., Monea,
S., Onisto, M., Garbisa, S., and Mignatti, P. (1997) EMBO J.,
16, 2319-2332.
32.Ramos-DeSimone, N., Hahn-Dantona, E., Sipley, J.,
Nagase, H., French, D. L., and Quigley, J. P. (1999) J. Biol.
Chem., 274, 13066-13076.
33.Falcone, D. J., McCaffrey, T. A., Mathew, J.,
McAdam, K., and Borth, W. (1995) J. Cell Physiol., 164,
334-343.
34.Konakova, M., Hucho, F., and Schleuning, W. D.
(1998) Eur. J. Biochem., 253, 421-429.
35.Flaumenhaft, R., and Rifkin, D. B. (1992) Mol.
Biol. Cell., 3, 1057-1065.
36.Naldini, L., Tamagnone, L., Vigna, E., Sachs, M.,
Hartmann, G., Birchmeier, W., Daikuhara, Y., Tsubouchi, H., Blasi, F.,
and Comoglio, P. M. (1992) EMBO J., 11, 4825-4833.