Mitochondria Enter the Nucleus (One Further Problem in Chronic Alcoholism)
L. E. Bakeeva1, V. P. Skulachev1*, Yu. V. Sudarikova2, and V. G. Tsyplenkova2
1Department of Bioenergetics, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-0338; E-mail: skulach@genebee.msu.su2Russian Cardiology Complex, Ministry of Health and Medical Industry, Moscow, Russia
* To whom correspondence should be addressed.
Received September 6, 2001
Electron microscopy of cardiomyocytes of patients with hypertrophic and alcoholic cardiomyopathies revealed the presence of nuclei with mitochondria accumulated in their core. This was associated with chromatin displacement to periphery of the nucleus. No large-scale intermixing of the nuclear content with the cytosol was found, although in some sections there were disruptions in the nuclear envelop continuity. The entrance of mitochondria into the nucleus was modeled in rats that were given ethanol and the catalase inhibitor aminotriazole for 12 weeks. It is suggested that the entrance of mitochondria into the nucleus promotes both the attack of mitochondria by nuclear proteins and the attack of nuclear DNA and proteins by proteins of the mitochondrial intermembrane space.
KEY WORDS: mitochondria, nucleus, alcoholic cardiomyopathy, electron microscopy
Abbreviations: AIF) apoptosis-inducing factor.
In 1958 Australian specialists in electron microscopy, H. Hoffman and G.
W. Grigg, reported a striking finding. In their studies on ultrathin
sections of adult mouse lymph nodes they found a mitochondrion inside
the cell nucleus. They suggested that mitochondria should be initially
generated in the nucleus and leave for the cytosol only afterwards [1]. This hypothesis was not confirmed. As to the
phenomenon itself, it seemed to be independently reopened in 1960 by a
Japanese scientist, H. Mori, who described the presence of mitochondria
in cells of four types of ascitic cancer and also in cells of tongue
and pancreas cancer and in regenerating liver cells of the newt [2]. The works cited were published in Exp. Cell
Res. and in Fukushima J. Med. Sci., respectively, and they
failed to get a broad response. The priority in mitochondria detection
inside the nucleus is usually ascribed to D. Brandes et al. who
published in 1965 in Science a brief note under the title of
“Nuclear Mitochondria?”, which was not supplemented with
references. In this case the authors dealt with leukemia cells L1210
[3]. During the next ten years a number of
publications appeared that showed the possibility of taking off the
question mark from the title of the Brandes article. Mitochondria have
been found in blood lymphocytes [4, 5], lymph nodes of patients with Hodgkin's disease [6], leukemia myoblasts [7], and
cardiomyocytes of a patient with rheumatic carditis [8].
Several interpretations were suggested for these findings. Usually it was assumed that the mitochondria are captured by the nucleus during its formation within the post-mitotic phase of the cell cycle or an artifact in tissue preparation and fixation. The first explanation is not acceptable in the case of cardiomyocytes, which extremely seldom if ever enter mitosis [9]. However, Norwegian authors described a patient with rheumatic carditis with mitochondria in 2-3% of the cardiomyocyte nuclei [8]. This work also cast doubt on the second interpretation (the fixation artifact) because no mitochondria were found in the cardiomyocyte nuclei of 11 other patients subjected to cardiosurgery [8].
The idea on the appearance of mitochondria in the nuclei of cardiomyocytes as a result of a particular heart pathology was supported in 1993 by one of the authors of this article (V. G. Tsyplenkova) by electron microscopy data on heart biopsy material taken from patients with hypertrophic cardiomyopathy [10]. A similar effect was also described by Takemura et al. [11] five years later.
The present article describes cases of the appearance of mitochondria in the cardiomyocyte nuclei of patients with hypertrophic and alcoholic cardiomyopathies. An experimental model of “alcoholic” rats is proposed for studies on such phenomena in animals.
MATERIALS AND METHODS
Biopsy material from the left ventricle endomyocardium was used that was taken during coronary angiography and ventriculography in patients with a clinical diagnosis of hypertrophic or alcoholic cardiomyopathy.
Experimental alcoholic cardiomyopathy was modeled in 160-180 g male Wistar rats by the method of Kino [12]. The animals were maintained on a liquid ethanol diet from which the alcohol provided 36% of the total calorific value of the food consumed. Concurrently, the animals were on alternate days injected intraperitoneally with 3-amino-1,2,4-triazole, a specific inhibitor of catalase activity, to increase the oxidative stress associated with the alcoholic intoxication. The animals were sacrificed 12 weeks after the experiment beginning. Ethanol was excluded from the diet the day before.
The material was fixed with 2.5% glutaraldehyde in phosphate buffer, post-fixed with osmic acid, dehydrated in alcohol solutions of increasing concentrations, and mounted in epoxide. Ultrathin serial sections were prepared using an LKB-IV microtome (Sweden), contrasted with uranyl acetate and lead citrate, and scanned with JEM-100CX and Hitachi-12 electron microscopes (Japan) at accelerating voltage of 80 and 75 kV, respectively.
RESULTS AND DISCUSSION
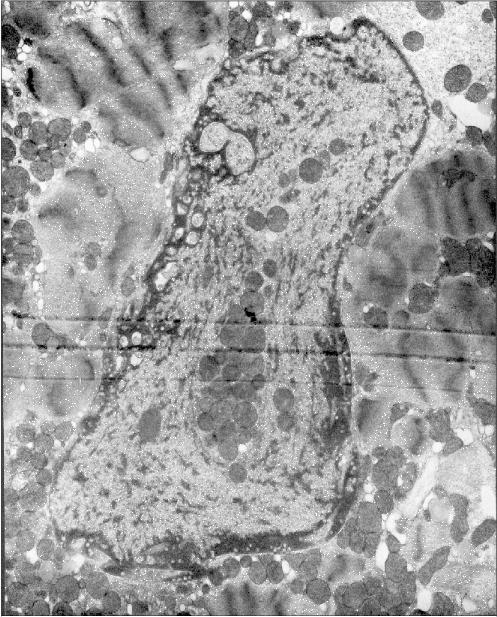
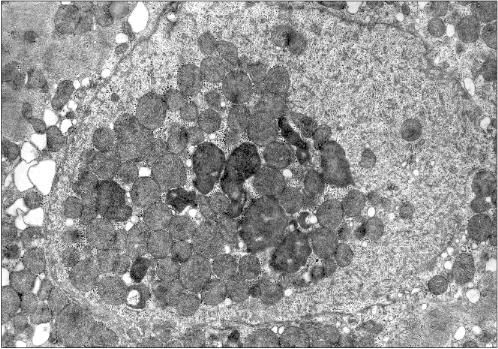
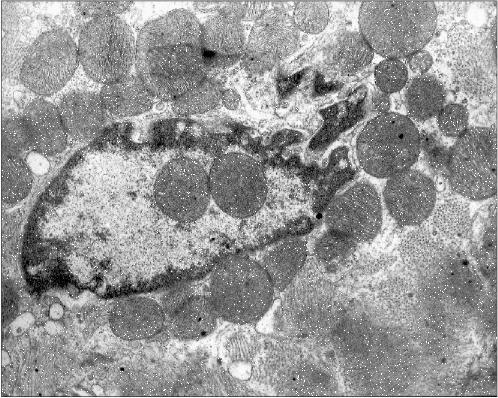
Figure 1 presents three patients' cardiomyocyte nuclei that contain mitochondrial clusters. Two patients were with hypertrophic cardiomyopathy and the third patient had alcoholic cardiomyopathy. One can see that the mitochondria are not separated by the nuclear membrane from the nuclear contents. This means that mitochondria are present in the nucleoplasm and not in a nuclear envelop invagination. All three microphotographs show the continuity of the nuclear membrane that separates the nuclear interior (with the mitochondria) from the cytosol.
Fig. 1a. Intranuclear location of mitochondria in cardiomyocytes of patients with heart failure: patient G. (hypertrophic cardiomyopathy); ×48,000.
Fig. 1b. Intranuclear location of mitochondria in cardiomyocytes of patients with heart failure: patient Sh. (hypertrophic cardiomyopathy); ×52,300.
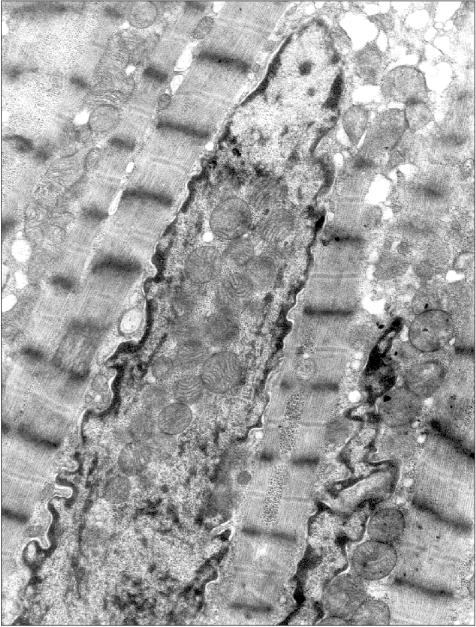
Figure 2 presents another microphotograph of a section through a cardiomyocyte of the patient with alcoholism. In this case the section clearly shows a large disruption of the nuclear envelop continuity and the mitochondria directed into the nucleus. One can also see that the nucleus contents still retains its characteristic morphology, as if it has not yet splashed out into the cytosol, being held inside the nucleus by certain forces despite the loss in the nuclear envelop continuity.Fig. 1c. Intranuclear location of mitochondria in cardiomyocytes of patients with heart failure: patient Kh. (alcoholic cardiomyopathy); ×73,400.
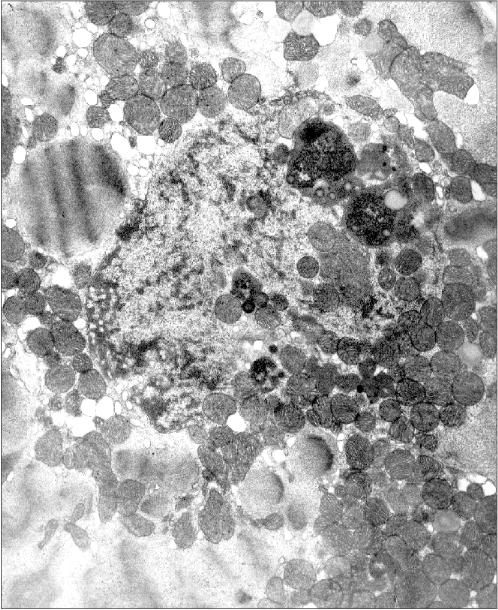
Figure 3 presents a section of a cardiomyocyte from a rat that was given ethanol and the catalase inhibitor aminotriazole for 12 weeks. Two mitochondria are clearly seen inside the nucleus. It should be emphasized that this model is well reproducible: five animals were treated as described above, and in four of them mitochondria were found inside the nuclei. This allows one to analyze the mechanism of such a phenomenon because in the previous studies the uniqueness of the object of study was a significant obstacle: the phenomenon was observed only in single cases. It should also be added that even in cases when intranuclear mitochondria were detected, the overwhelming majority of sections across nuclei did not reveal mitochondria. Thus, it is easy to understand why the mechanism and probable functional implications of this phenomenon remained virtually unexplored for more than 40 years.Fig. 2. A section across the nucleus of a cardiomyocyte of the patient with alcoholic cardiomyopathy; ×58,700.
It seems virtually doubtless that mitochondria are undesirable guests for the nucleus. Mitochondria are a source of reactive oxygen species that are capable of oxidizing DNA. Moreover, they are a depot of apoptosis-activating proteins that initiate the reaction cascade finally resulting in the degradation of nuclear DNA and also of lamin and some other proteins of the nucleus. However, it is likely that just the last circumstance can be the clue for understanding the intranuclear mitochondria phenomenon.Fig. 3. Intranuclear location of mitochondria in cardiomyocytes of an “alcoholic” rat; ×95,200.
Examination of microphotographs of the mitochondria-containing nuclei shows that in most cases we are dealing with the cells that are entering apoptosis. Thus, most of the figures in the present article show chromatin margination (the accumulation at the edges of the nucleus) that is typical for apoptosis.
From our data and the data of other laboratories [13, 14], apoptosis is associated with the disintegration of initially long mitochondrial filaments and their networks to small rounded mitochondria that move towards the nucleus. This movement is associated with a selective inactivation of kinesin, a motor protein responsible for the centrifugal movement of intracellular particles, whereas dynein, a motor providing particle movement to the center remains active [14]. One may suggest that at least in some cases the penetration of mitochondria into the nucleus should be the concluding stage of their centripetal movement. In the nucleus, the mitochondria are likely to release the apoptosis-inducing factor (AIF), which activates a special nuclease attacking nuclear DNA. The releasing of this factor, which is normally sequestered in the mitochondrial intermembrane space, seems to be stimulated by the translocation of nuclear proteins p53 [15] and TR3 [16] from the nucleus into the mitochondria. The nuclear proteins cause the opening of pores in the mitochondrial inner membrane, the swelling of their matrix, and, as a consequence, disruption of the outer mitochondrial membrane with the releasing of the proteins of the intermembrane space into the cytoplasm. Thus, the movement of mitochondria towards the nucleus and then into the nucleus can be considered to be a manner of (a) transfer into the nucleus of apoptosis-activating proteins of AIF type and (b) promotion of the attack of mitochondria by nuclear proteins such as p53 and TR3. In this connection, it seems interesting to study apoptosis during early embryogenesis; the latter was recently shown [17] to be specifically activated by AIF, i.e., by the intermembrane space protein that does not need cytosolic proteins to realize its proapoptotic effect. (This is the difference of AIF from cytochrome c, another protein of the intermembrane space, which activates apoptosis through cytoplasmic proteins Apaf-1 and caspase 9 and 3 [18]).
In remains unclear how the nuclear membrane is opened to allow the entrance of mitochondria into the nucleus ([8, 11] and Fig. 2 of this article). It is unlikely to be the result of a simple mechanical injury to the nucleus due to blebbing, which the cell undergoes during apoptosis, because the contents of the nucleus and the cytoplasm are not intermixed, as mentioned above. We suggest more likely a short-term opening of a small area of the nuclear envelope with a subsequent “healing” of the hole occurring after the mitochondria have entered the nucleus and formed a cluster in its central part.
The work was supported by the Russian Foundation for Basic Research (projects Nos. 97-04-49149 and 01-04-48606), by the Leading Scientific Schools (00-15-97799), and by the Ludwig's Cancer Institute (RBO-863).
REFERENCES
1.Hoffman, H., and Grigg, G. W. (1958) Exp. Cell
Res., 15, 118-131.
2.Mori, H. (1960) Fukushima J. Med. Sci.,
7, 21-32.
3.Brandes, D., Schonfield, B. H., and Antom, E.
(1965) Science, 149, 1373-1374.
4.Klug, H. (1966) Naturwissenschaften,
53, 339.
5.Bloom, G. D. (1967) J. Cell Biol.,
35, 266-268.
6.Oliva, H., Valle, A., Diaz Flores, L., and Rivas,
M. C. (1973) Virchows Arch. Abt. B. Zellpath., 12,
189-194.
7.Schumacher, H. R., Szekely, I. E., Patel, S. B.,
and Fisher, D. R. (1974) Am. J. Pathol., 74, 71-82.
8.Jensen, H., Engedal, H., and Selmer Satersdal, T.
(1976) Virchows Arch. B. Cell Path., 21, 1-12.
9.Quiani, F., Cigola, E., Lagrasta, C., Saccani, G.,
Quiani, E., Rossi, C., Olivetti, G., and Anversa, P. (1994) Circ.
Res., 75, 1050-1063.
10.Tsyplenkova, V. G., and Beskrovnaya, N. N. (1993)
Arkhiv Patol., 55, 26-29.
11.Takemura, G., Takatsu, Y., Sakaguchi, H., and
Fujiwara, H. (1997) Pathol. Res. Pract., 193,
305-311.
12.Kino, M. (1981) J. Mol. Cell. Cardiol.,
13, 5-21.
13.Skulachev, V. P. (2001) Trends Biochem.
Sci., 26, 23-29.
14.De Vos, K., Severin, F., van Herreweghe, F.,
Goossens, V., Hyman, A., and Grooten, J. (2000) J. Cell Biol.,
149, 1207-1214.
15.Marchenko, N. D., Zaika, A., and Moll, U. M.
(2000) J. Biol. Chem., 275, 16202-16212.
16.Li, H., Kolluri, S. K., Gu, J., Dawson, M. I.,
Cao, X., Hobbs, P. D., Lin, B., Chen, G.-Q., Lu, J.-S., Lin, F., Xie,
Z., Fontana, J. A., Reed, J. C., and Zhang, X.-K. (2000)
Science, 289, 1159-1164.
17.Joza, N., Susin, S. A., Daugas, E., Stanford, W.
L., Cho, S. K., Li, C. Y. J., Sasaki, T., Elia, A. J., Cheng, H.-Y. M.,
Ravagnan, L., Ferri, K. F., Zamzami, N., Wakeham, A., Hakem, R.,
Yoshida, H., Kong, Y.-Y., Mak, T. W., Zuniga-Pflucker, J. C., Kroemer,
G., and Penninger, J. M. (2001) Nature, 410, 549-554.
18.Skulachev, V. P. (2001) Exp. Gerontol.,
36, 995-1024.