Apoptosis in the Initial Leaf of Etiolated Wheat Seedlings: Influence of the Antioxidant Ionol (BHT) and Peroxides
V. A. Zamyatnina, L. E. Bakeeva, N. I. Aleksandrushkina, and B. F. Vanyushin*
Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3181; E-mail: vanyush@belozersky.msu.ru* To whom correspondence should be addressed.
Received August 23, 2001; Revision received September 20, 2001
Apoptosis was observed in the initial leaf of 5-8-day-old etiolated wheat seedlings. A condensation of cytoplasm in apoptotic cells, formation of myelin-like structures, specific fragmentation of cytoplasm, appearance in vacuoles of specific vesicles containing subcellular organelles, condensation and margination of chromatin in the nucleus, and internucleosomal fragmentation of nuclear DNA are ultrastructural features of apoptosis in the initial wheat leaf. Single-membrane vesicles detected in vacuoles of the leaf cells resemble in appearance the vacuolar vesicles in the coleoptile apoptotic cells described earlier (Bakeeva, L. E., et al. (1999) FEBS Lett., 457, 122-125); they contain preferentially plastids but not mitochondria as was observed in coleoptile. The vacuolar vesicles are specific for the apoptotic plant cells. Thus, apoptosis in various tissues is an obligatory element of plant (wheat) growth and development even in the early stages of ontogenesis. Contrary to strong geroprotecting action in coleoptile, the known antioxidant BHT (ionol, 2.27*10-4 M) does not prevent in the leaf cells the apoptotic internucleosomal DNA fragmentation and appearance of specific vacuolar vesicles containing subcellular organelles. Therefore, the antioxidant action on apoptosis in plants is tissue specific. Peroxides (H2O2, cumene hydroperoxide) stimulated apoptosis (internucleosomal DNA fragmentation) in coleoptile and induced it in an initial leaf when apoptosis in a control seedling leaf was not yet detected. Thus, apoptosis that is programmed in plant ontogenesis and controlled by reactive oxygen species (ROS) can be modulated by anti- and prooxidants.
KEY WORDS: aging, antioxidant, apoptosis, BHT, cell ultrastructure, DNA fragmentation, leaf, ontogenesis, peroxides, plant, plastid differentiation, wheat
Abbreviations: BHT) butylated hydroxytoluene, or 2,6-di-tert-butyl-4-methylphenol, or ionol; mtDNA) mitochondrial DNA; nDNA) nuclear DNA; ROS) reactive oxygen species.
Embryogenesis and other early stages of ontogenesis in plants, similarly
to animals, are accompanied with programmed death of individual cells
and tissues [1, 2]. The
selective knockout of AIF genes coding for apoptogenic proteins
prohibits embryogenesis in animals at very early stages [3]. Apoptotic elimination of cells is an indispensable
condition of plant development [1]. In particular,
apoptosis was observed in developing barley anthers [4], endosperm of forming corn seed [5], barley aleurone layer [6],
endosperm of germinating castor beans [7],
coleoptile of developing wheat seedlings [8], and
many other tissues and cells in various plants [9-11].
Apoptosis in plants is manifested in a set of specific changes in cell structure and morphology: chromatin condensation [10] with subsequent decay of the nucleus and internucleosomal nDNA fragmentation [8, 12], huge vacuoles are formed in the cells [8, 13, 14]. In plants the destruction of tonoplast and vacuolization of cytoplasm usually precede the destruction of the nucleus and mitochondria [14, 15]. In contrast to animals, the apoptotic cells in plants due to solid cell walls, do not disappear completely, and they take part in formation of vascular bundles and aerenchyma [14, 15].
Cereal seedlings are unique and very useful model for investigation of apoptosis in plants. Coleoptile in cereals functions for a relatively short period at the early stages of ontogenesis, and it dyes quickly as the seedling grows and develops. The apoptotic fragmentation of nuclear DNA in this organ was detected in 6-day-old etiolated wheat seedlings, and it is expressed very strongly in 8-day-old seedlings [8, 12]. This is accompanied by strong reorganization of the cytoplasm; in particular, specific single-membrane vesicles containing mitochondria actively replicating mtDNA are formed in the cellular vacuole [11, 12]. These distinctive events in the nucleus and cytoplasm serve as reliable markers of apoptosis in plants.
In 8-day-old wheat seedlings internucleosomal DNA fragmentation was clearly observed also in the initial leaf, but it was detected only in the apical (i.e., most senescent) leaf part [12]. Detection of the internucleosomal DNA fragmentation in the initial leaf could testify to the fact that along with the aging coleoptile apoptosis seems to appear also in the developing initial leaf. But this conclusion was quite unexpected and even unlikely because apoptosis was observed only in senescent but not young leaves [16, 17]. Besides, it is known that apoptosis is typical for leaf aging and dying [17]. To be sure that we really deal with the apoptosis in an initial leaf that is very young and developing organ of wheat seedling, along with nuclear DNA fragmentation observed [12] some other evidence of apoptosis at the level of the ultrastructural organization of the cell was also needed. In particular, it was important to find whether apoptosis in the initial leaf is accompanied by condensation and margination of chromatin and formation of specific vacuolar vesicles containing organelles detected earlier in aging coleoptiles [11, 12]. All these features in common are considered as the best evidence of apoptosis.
It is known that oxidative status in the cell controls mitosis and apoptosis [1, 2, 12, 18]. Reactive oxygen species (ROS) are needed for plant development [12, 19, 20], and they can trigger apoptosis [12, 18]. We earlier observed that apoptosis in wheat coleoptile was completely blocked by the antioxidant BHT (ionol) [12]. If, in fact, apoptosis takes place in the initial leaf, it is important to also learn to what extent it can be modulated there by antioxidants and oxidants. Besides, it was interesting to determine whether BHT influences the apoptosis in the initial leaf in a similar fashion as it was doing in coleoptile.
In the present work, we tried to detect the specific ultrastructural features of apoptosis in the initial leaf of developing etiolated wheat seedlings and to study how the antioxidant BHT (ionol) and peroxides (H2O2, cumene hydroperoxide) may influence them.
MATERIALS AND METHODS
Seeds of Mironovskaya 808 variety of winter wheat (Triticum aestivum L.) were germinated in darkness for 24 h at 26°C on wet filter paper in a plastic cuvette; sprouted seeds were transferred into other cuvette, covered with a lid, and grown for 24 h in darkness at 26°C. Then, for the experimental plants, water as the medium was changed for BHT solution (Sigma, USA; 50 mg/liter, 2.27*10-4 M) or peroxides (H2O2, cumene hydroperoxide, 10-3 M) (Aldrich, USA), and the growth of the seedlings was continued in darkness at 26°C for a few days. Thus, the whole plant was exposed to the compounds used and their effects were judged from analysis of the separated plant organs (coleoptile, initial leaf). The reagent solutions used were changed once a day for freshly prepared solutions. To prepare BHT water solution, solid BHT was dissolved in ethanol and added to boiling water to the concentration required, then the weakly opalescent mixture was cooled to room temperature. An equivalent volume of ethanol was added to the water used for growing the control wheat seedlings.
Seedlings of defined age (seedling age was estimated in days starting from the beginning of the seed soaking time) were thoroughly washed with water; coleoptiles and initial leaves were separated and used for determination of DNA isolation. Each experiment (plant growing in the presence of each compound tested at a particular concentration) was done at least twice and accompanied by an independent control (plant growing in water under the same conditions).
To isolate DNA, the coleoptiles or leaves of the etiolated wheat seedlings were thoroughly ground in a mortar and pestle in liquid nitrogen, a lysing solution (50 mM Tris-HCl, pH 7.5, 25 mM EDTA, 1% SDS) was added to the fine powder obtained, and the mixture was then incubated for 30 min at room temperature. Then NaCl was added to 1 M concentration, and the mixture was deproteinized by careful shaking with chloroform-isoamyl alcohol mixture (10 : 1 v/v). After centrifugation for 10 min at 5000g, DNA was precipitated from the aqueous phase by addition of three volumes of 96% ethanol and dissolved then in 50 mM Tris-HCl, pH 7.5, containing 25 mM EDTA. The DNA samples obtained were treated with DNase-free ribonuclease A (50 µg/ml) for 20 min at 37°C, and DNA was precipitated again with addition of three volumes of 96% ethanol.
Similar aliquots of isolated and purified DNA preparations were subjected to electrophoresis for 2 h in 1.2% agarose gels at 2-3 V/cm in 0.09 M Tris-borate buffer, pH 8.3, containing 0.5 µg/ml ethidium bromide.
Fixation of intact seedlings for the electron microscopic analysis was done in 25% glutaraldehyde and 2% OsO4 solution. Seedlings were cut from the seeds; coleoptile and leaf were separated at 0°C in a fixing solution prepared in 0.1 M buffer, pH 7.2 (0.1 M Na2HPO4 and 0.1 M KH2PO4). Twenty milliliters of the phosphate buffer, 300 mg sucrose, 3 ml 25% glutaraldehyde, and 0.5 ml 40% formaldehyde were used for preparation of the fixing solution. After vacuum treatment, the material was left in a fixing solution at room temperature for 1.5-2 h. Then the fixing solution was poured off and the material was incubated for 30 min in a freshly prepared buffer, samples were incubated then in 2% OsO4 solution for 1.5 h (second fixation). The material was dehydrated then with ethanol (increasing concentrations). The fixed material was stored at 2°C. After dehydration the sections were placed into acetone-Epon 812 mixture with gradual increase in the Epon 812 concentration (2 : 1, incubation for 3 h; 1 : 1, incubation for 24 h; 1 : 2, incubation for 24 h at room temperature). The material was finally placed in Epon 812 and incubated for 24 h at 37°C and then for 5 days at 60°C.
Sections obtained with an LKB-III Microtome (Sweden) were stained according to Reynolds and analyzed with Hitachi-11 or Hitachi-12 electron microscopes (Japan).
RESULTS AND DISCUSSION
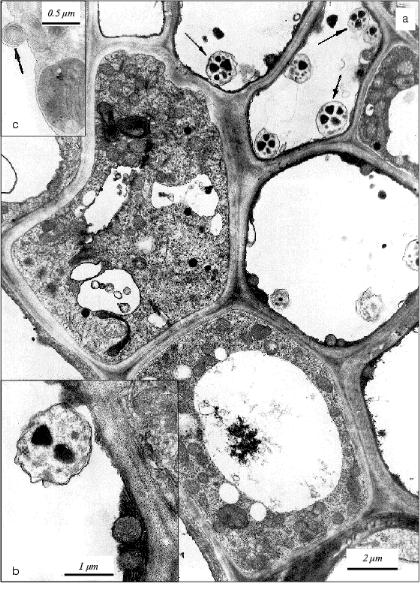
Ultrastructural features of parenchyma (apoptotic) cells in apical area of the initial leaf of etiolated wheat seedlings. The collection of cells with obvious ultrastructural features of apoptosis were observed by electron microscopy in sections of the apical part of the initial leaf in 5-day-old etiolated wheat seedlings. A large central vacuole and narrow electron-dense wall-located tonoplast layer are specific for such cells (Fig. 1). The cytoplasm in these cells does not have any visible signs of destruction; it contains many elements of Golgi apparatus, endoplasmic reticulum, and all main cellular organelles--nuclei, mitochondria, plastids, and ribosomes. Ribosomes are represented by polyribosomal complexes or they are associated with membranes of the endoplasmic reticulum. The main population of plastids in these cells is represented by chloroplasts with relatively well-developed thylakoid system. Amyloplasts were also observed among plastids. In the cytoplasm of these cells, we observed mitochondria with electron-dense structure; similar mitochondria are specific for apoptotic animal cells [21]. The condensed ultrastructure of mitochondria in the cells of the initial leaf of wheat seedling may also be considered as an index of the developing apoptosis. Besides, myelin-like structures appear in the cytoplasm of the cells of the apical part of the initial leaf in 5-day-old seedlings (Fig. 1). This testifies also to development of the aging process in the initial leaf.
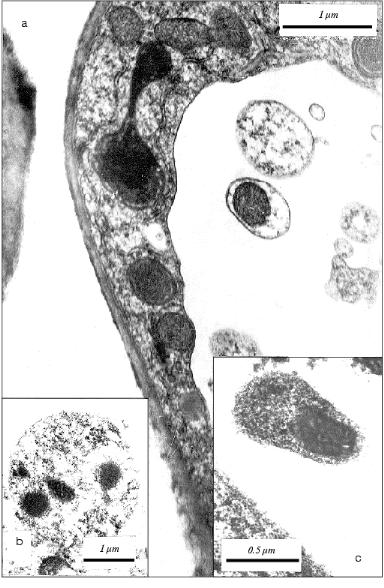
Formation in the vacuole of specific vesicles with mitochondria actively synthesizing DNA [11] is a characteristic feature of the apoptotic cells in aging coleoptile of etiolated wheat seedlings [11, 12]. We detected first similar vesicles in situ in vacuoles of apoptotic cells in the initial leaf of the wheat seedlings (Fig. 1). Similarly to vesicles in coleoptile apoptotic cells [11, 12], they are represented by single-membrane formations containing subcellular organelles. In the leaf apoptotic cells these vesicles contain mainly plastids (Fig. 1) but not mitochondria as in the case in the cells of aging coleoptile [11, 12]. Vesicles containing mitochondria were observed also in the initial leaf (Fig. 2), but they are less frequent there compared with that in coleoptile [11, 12]. It is worth mention that vesicles containing plastids were observed only in the apoptotic cells with narrow tonoplast layer or almost without tonoplast.Fig. 1. Ultrastructure of parenchyma cells in the leaf apical part of a 5-day-old etiolated wheat seedling: a) apoptotic cells with narrow wall-located layer of cytoplasm containing electron-dense mitochondria are seen. Vacuolar cytoplasmic vesicles containing plastids are shown by arrows; b) fragment of apoptotic cell neighboring the cell with normal structure. Mitochondria with increased electron density are distinguished in the narrow wall-located electron-dense layer of cytoplasm. A cytoplasmic vesicle containing a plastid is seen in the vacuole of the apoptotic cell; c) fragment of parenchyma cell containing myelin-like structure (shown by arrow).
Thus, formation in the cellular vacuole of vesicles with subcellular organelles is a specific feature that is common for apoptotic plant cells. It occurs in very functionally different and specialized plant organs (tissues) such as leaf and coleoptile.Fig. 2. Fragments of parenchyma cells of the apical part of the initial leaf of etiolated wheat seedlings of various age: a) fragment of the leaf parenchyma cell of 5-day-old wheat seedling. A vesicle containing electron-dense mitochondrion is seen in the vacuole; b) a vesicle with electron-dense mitochondria in vacuole in the cell of an initial leaf of an 8-day-old etiolated wheat seedling grown in the presence of 50 mg/ml BHT; c) a vesicle containing an electron-dense mitochondrion in the vacuole of a cell in an initial leaf of an 8-day-old etiolated wheat seedling grown in water (control plant).
This amazing event observed in the apoptotic plant (leaf) cell as well as detected and described earlier by us in coleoptile [11]--the separation of some parts of the cytoplasm with organelles as closed single-membrane vesicles and their escape into cellular vacuole--seems to some extent similar to blebbing of the apoptotic animal cells [21]. The fragmentation of the cytoplasm is one of the main ultrastructural features of apoptosis in animals. But this similarity is only apparent. In contrast to blebs as elements of rapid reorganization and decay of the cytoplasm in animals, the vacuolar vesicles in an apoptotic plant cell seem to play other functional role. First of all, the plant vesicles observed differ from all known specific cellular depots where degradation of cellular structures and biopolymers takes place. We never observed any degradation marks of the subcellular structures located in these Ca2+-enriched vesicles in the coleoptile cells [11]. There are no degradation signs also in plastids and mitochondria observed in the vacuolar vesicles in the cells of the initial leaf (Figs. 1 and 2). It seems that the condition for the organelle survival in vesicles is even more comfortable than that in other cytoplasmic compartments of the apoptotic plant cell.
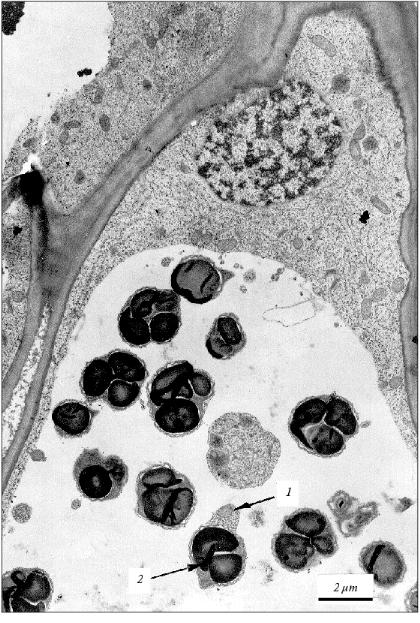
The vesicles with mitochondria detected in vacuoles of the leaf cells (Fig. 2) are analogous to those in vacuoles of the coleoptile cells (Fig. 3). We isolated earlier the fraction of vacuolar vesicles from wheat coleoptiles and showed that mitochondria in these vesicles have intact structure and actively consume oxygen [11, 22]. This obviously cannot be a property of degenerating subcellular organelles. Besides, active replication of mtDNA in the cells of aging coleoptile takes place exactly in these vesicles [11, 22-25] in spite of concomitant degradation of the apoptotic cells. Therefore, we can assume that the vesicles observed seem to shelter and preserve some share of segregated organelles, at least, for some period during the process of the apoptotic death of the cell. One cannot rule out that the strong replication of mtDNA in vesicles observed earlier [11, 22] is due to alienation of vesicular mitochondria from the nucleus compared with free (non-vesicular) mitochondria and loss of the nuclear control for replication of these mtDNA. But the true sense and possible biological significance of these peculiar events are still unknown.
Anyway, in the cells of aging coleoptile and developing initial leaf of wheat seedlings the individual populations of DNA-containing organelles are formed due to the fragmentation of the cytoplasm in the process of apoptosis. These organelles (mitochondria) are able to synthesize their own DNA [11] under conditions of DNA degradation in the nucleus. The material of degrading nuclear DNA seems to be used for the synthesis of these mtDNA. It might be that some part of the mitochondrial and plastid DNA newly formed in vesicles of the agonizing apoptotic cell does not perish with this cell but somehow migrates to neighboring cells. In this particular case, the known age (apoptotic) superproduction of mtDNA in plants [22-25] could be quite appropriate. The transposition of mtDNA (they are mainly plasmid-like cyclic molecules of various contour lengths [25]) or plastid DNA from cell to cell has not yet been described. Nevertheless, this cannot be ruled out because plant cells are easily transformed by bacterial plasmid DNA and active intercellular transport of viral infections occurs in plants.Fig. 3. Fragment of the parenchyma cell of the coleoptile apical part of 3-day-old etiolated wheat seedling. Vesicles containing subcellular organelles are seen in a vacuole. Mitochondrion (1) and plastid (2) present in a single vacuolar vesicle are shown by arrows.
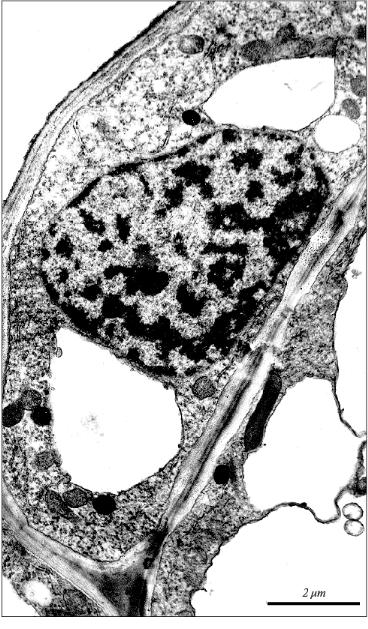
Condensation and margination of chromatin are well known specific ultrastructural features of apoptosis in animals [21]. We observed marked condensation and margination of chromatin in the nuclei of apoptotic cells in the apical part of the initial leaf in 5-day-old etiolated wheat seedlings (Fig. 4). Similar condensation and margination of chromatin was detected earlier in wheat coleoptile (in the nuclei of cells with vacuolar vesicles). These specific apoptotic changes in the chromatin organization precede the domain and internucleosomal fragmentation of nuclear DNA [1].
Thus, there is no doubt that development of the initial leaf even in very young (5-day-old) etiolated wheat seedlings is accompanied by apoptosis. This process becomes apparent relatively diffusely as the original apoptotic centers neighbor leaf cells with normal morphology. Distinct ultrastructural features of apoptosis in the initial leaf of etiolated wheat seedlings are: 1) compaction of cytoplasm in the apoptotic cell; 2) formation in the cell of myelin-like structures that certifies the beginning of the aging process in the leaf; 3) specific fragmentation of cytoplasm; 4) an appearance in the vacuole of specific single-membrane vesicles containing organelles; 5) condensation and margination of chromatin in the nucleus; 6) internucleosomal fragmentation of nuclear DNA. These specific features for the apoptotic cells observed by us in developing wheat seedlings seem to serve as markers of apoptosis in plants in general.Fig. 4. Picture of apoptotic condensation and margination of chromatin in the nucleus of parenchyma cell in the apical part of the initial leaf of a 5-day-old etiolated wheat seedling.
Therefore, the aging of leaves in cereals, as in other plants [16, 17], proceeds by apoptosis, and it starts to appear even at the very early stages of ontogenesis. According to our electron microscopic data some marks of the aging of the leaf cells (appearance of myelin-like structures in the cytoplasm of the cells of the initial leaf) were observed even in 5-day-old etiolated wheat seedlings.
Effect of antioxidant ionol (BHT) and peroxides on apoptosis in the initial leaf of etiolated wheat seedlings. Induction and progress of apoptosis in plants in many respects depend on the intracellular oxidative status [1, 18]. The development of the etiolated wheat seedlings is accompanied by cyclic formation of superoxide anion that is essential for plant growth and development [20]. The growth and development of plants can be effectively regulated by modulation of oxidative status with anti- and prooxidants [12, 19] because the reactive oxygen species as secondary messengers control apoptosis, mitosis, and differentiation of cells, plastids, and other subcellular organelles [1, 12].
We found that the antioxidant ionol (BHT) at concentration 50 mg/liter (2.27*10-4 M) inhibits growth of etiolated wheat seedlings, changes morphology of their organs, prolongs the coleoptile life-span, and prevents the appearance of specific marks of aging [12]. In particular, BHT prevents in coleoptile the age-dependent decrease in the content of total DNA and protein [20], the apoptotic internucleosomal fragmentation of nuclear DNA, the appearance in cellular vacuoles of specific vesicles with mitochondria actively replicating mtDNA and the formation in them of heavy (rho = 1.718 g/cm3) mtDNA [12]. BHT induces formation of chloroplasts in the roots of etiolated seedlings [12].
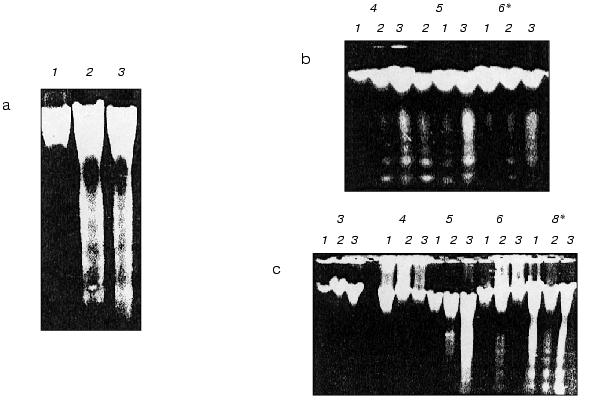
Contrary to coleoptile, in the initial leaf of 8-day-old etiolated wheat seedling BHT does not inhibit formation in the cellular vacuole of specific apoptotic vesicles containing subcellular organelles (Fig. 2b) and it does not block internucleosomal apoptotic DNA fragmentation in the nucleus (Fig. 5a, lane 2). Similarly to control 8-day-old seedlings (Fig. 2c), we observe the specific vesicles containing mitochondria in the cellular vacuoles in initial leaf of the same age seedlings grown in the presence of BHT (Fig. 2b). These vesicles occur there even more often than in the leaf of the control seedlings grown in the absence of BHT. Thus, the effect of BHT on apoptosis in etiolated wheat seedlings has tissue-specific character. According to our data, in contrast to coleoptile, BHT does not inhibit but seems even slightly stimulates apoptosis in the initial leaf. It is more probable that this is due to different concentration of intracellular oxygen in the leaf and coleoptile. Despite the fact that the plants were grown in darkness, they were taken out the darkness from the thermostat for relatively short periods (few minutes) once a day when water or respective solutions of chemical were changed; these short light exposures were probably efficient enough for some photosynthesis producing oxygen in the leaf cells (the initial leaf in seedlings grown under these conditions had weak yellow-greenish color compared with colorless coleoptile). At least, well differentiated plastids with developed membrane structure in the cells of the initial leaf were seen (Fig. 1). It was recently shown that BHT and its derivatives may have prooxidant properties under increased partial oxygen pressure [26, 27]. This may be due to the fact that under relatively high concentrations of molecular oxygen BHT is oxidized by a single electron mechanism to the respective carcinogenic BHT-quinone that generates superoxide-anion under specific conditions [28]. This seems to be responsible for a significant carcinogenic effect of BHT in animal tissues enriched with oxygen such as lungs and others [26, 27]. Besides, it may to some extent explain the fact that in the same concentration BHT has less retardant effect on the wheat seedlings grown in the light (under condition of high formation of oxygen due to photosynthesis) compared with plants grown in darkness [12]. Thus, BHT can act as antioxidant as well as prooxidant both in plant and animal cells. Strong BHT action inducing plastid differentiation [12] and formation of pigments [19] in roots of etiolated wheat seedlings seems to be associated with its prooxidant activity and it is similar to the influence of typical prooxidants on the formation of carotenoids in the cells of pepper plants [29].
In contrast to BHT, the peroxides such as H2O2 and cumene hydroperoxide (10-3 M) unambiguously stimulated apoptosis (DNA fragmentation) in coleoptile and induced it in initial leaf when in leaf of control seedlings apoptosis was not yet detected (Fig. 5, b and c). We did not observe any changes in morphology of the seedlings grown in the presence of these peroxides.Fig. 5. Electrophoregrams of DNA isolated from initial leaves and coleoptiles of etiolated wheat seedlings of various age: a) DNA from coleoptile (1) and initial leaf (2) of 8-day-old wheat seedlings grown in the presence of 50 mg/liter (2.27*10-4 M) BHT; 3) DNA from an initial leaf of control 8-day-old seedlings grown in water; b) DNA from initial leaf of 4-6-day-old seedlings grown in water (1) (control) or in the presence of 10-3 M cumene hydroperoxide (2) or 10-3 M H2O2 (3); c) DNA from coleoptiles of 3-8-day-old seedlings grown in water (control) (1) or in the presence of 10-3 M cumene hydroperoxide (2) or 10-3 M H2O2 (3); * seedling age (days).
Strong internucleosomal fragmentation and degradation of coleoptile nDNA under the influence of hydrogen peroxide was found in 5-day-old wheat seedlings. It is interesting that at the same time the formation of endogenous superoxide-anion in the coleoptile cells is minimal [20]. It seems that “forcible” maintenance of high oxidative status by peroxides in a period of the natural minimal content of reactive oxygen species (ROS) is a supersensitive element of the triggering (intensification) of apoptotic DNA fragmentation. Plant sensitivity to induction of apoptosis by peroxides seems to be associated with the natural cycles of ROS formation in seedlings [20], and it is different at the various stages of early ontogenesis.
Peroxides (in the presence of Fe2+ or in the light they are the source of the reactive radical *OH [30, 31]) significantly increase the apoptotic DNA fragmentation both in the initial leaf and coleoptile (Fig. 5). Hydrogen peroxide and cumene hydroperoxide induce apoptosis (DNA fragmentation) already on the fourth day of the seedling life (Fig. 5b). In control plants the apoptotic DNA fragmentation in the initial leaf was observed only on the eighth day of the seedling life (Fig. 5a, lane 3). In a leaf H2O2 stimulates apoptosis approximately 24 h earlier (Fig. 5b) than in coleoptile (Fig. 5c). This seems to be due in part to higher oxygen and Fe2+ contents in photosynthesizing leaf than in coleoptile. As far as hydrogen peroxide speeds up the appearance of apoptosis in the initial leaf, it should promote leaf aging. It is known that peroxides induce aging in plants [1, 18]. In the initial leaf of etiolated wheat seedlings, as in their coleoptile, the “fifth day effect” characterized by strong stimulation by hydrogen peroxide of internucleosomal DNA fragmentation was observed exactly in this period of the seedling life (Fig. 5, b and c) when the natural level of the superoxide content in the cells of etiolated seedling was minimal [20].
Thus, modulation of the ROS level in seedling by peroxides and antioxidants results in tissue-specific change in the target date for the appearance as well as intensity of apoptosis that is an integral part of plant ontogenesis.
We greatly thank engineer V. V. Kruglyakov for technical assistance in electron microscopy.
This work was supported in part by the Russian Foundation for Basic Research (grant Nos. 99-04-48090, 00-15-97920, 01-04-48647, 01-04-06272, and 01-04-48606).
REFERENCES
1.Vanyushin, B. F. (2001) Usp. Biol. Khim.,
41, 3-38.
2.Greenberg, J. T. (1996) Proc. Natl. Acad. Sci.
USA, 93, 12094-12097.
3.Joza, N., Susin, S. A., Dauglas, E., Stanford, W.
L., Cho, S. K., Li, C. Y. J., Sasaki, T., Ella, A. J., Cheng, H.-Y. M.,
Ravagnan, L., Ferri, K. F., Zamzaml, N., Wakeham, A., Hakem, R.,
Yoshida, H., Kong, Y.-Y., Mak, T. W., Zuniga-Pfluecker, J. C., Kroemer,
G., and Penninger, J. M. (2001) Nature, 410, 549-554.
4.Wang, M., Hoekstra, S., van Bergen, S., Lamers, G.
E., Oppedijk, B. J., van der Heijden, M. W., de Priester, W., and
Schilperoort, R. A. (1999) Plant Mol. Biol., 39,
489-501.
5.Bethke, P. C., Lonsdale, J. E., Fath, A., Jones, R.
L., Young, T. E., and Gallie, D. R. (2000) Plant Mol. Biol.,
42, 397-414.
6.Wang, M., Oppedijk, B. J., Lu, X., van Duijn, B.,
and Schilperoort, R. A. (1996) Plant Mol. Biol., 32,
1125-1134.
7.Schmid, M., Simpson, D., and Gietl, C. (1999)
Proc. Natl. Acad. Sci. USA, 96, 14159-14164.
8.Kirnos, M. D., Aleksandrushkina, N. I., and
Vanyushin, B. F. (1997) Biochemistry (Moscow), 62,
864-869.
9.Xu, Y., and Hanson, M. R.(2000) Plant
Physiol., 122, 1323-1333.
10.O'Brien, I. E., Murray, B. G., Baguley, B. C.,
Morris, B. A., and Ferguson, I. B. (1998) Exp. Cell. Res.,
241, 46-54.
11.Bakeeva, L. E., Kirnos, M. D., Aleksandrushkina,
N. I., Kazimirchyuk, S. B., Shorning, B. Yu., Zamyatnina, V. A.,
Yaguzhinsky, L. S., and Vanyushin, B. F. (1999) FEBS
Lett., 457, 122-125.
12.Bakeeva, L. E., Zamyatnina, V. A., Shorning, B.
Yu., Aleksandrushkina, N. I., and Vanyushin, B. F. (2001)
Biochemistry (Moscow), 66, 850-859.
13.Groover, A., and Jones, A. M. (1999) Plant
Physiol., 119, 375-384.
14.Drew, M. C., He, I. I., and Morgan, P. W. (2000)
Trends Plant Sci., 5, 123-127.
15.Fukuda, H. (2000) Plant Mol. Biol.,
44, 245-253.
16.Yen, C. H., and Yang, C. H.(1998) Plant Cell
Physiol., 39, 922-927.
17.Nooden, L. D., Guiamet, J. J., and John, I.
(1997) Physiol. Plant., 101, 746-753.
18.Jabs, T. (1999) Biochem.
Pharmacol., 57, 231-245.
19.Shorning, B. Yu., Poleshchuk, S. V., Gorbatenko,
I. Yu., and Vanyushin, B. F. (1999) Izv. RAN, Ser. Biol.,
1, 30-38.
20.Shorning, B. Yu., Smirnova, E. G., Yaguzhinsky,
L. S., and Vanyushin, B. F. (2000) Biochemistry (Moscow),
65, 1357-1361.
21.James, T. N., Terasaki, F., Pavlovich, E. R., and
Vikhert, A. M.(1993) J. Lab. Clin. Med., 122,
309-323.
22.Kirnos, M. D., Aleksandrushkina, N. I., Bakeeva,
L. E., Kazimirchyuk, S. B., Shorning, B. Yu., Alekseeva, V. A.,
Yaguzhinsky, L. S., and Vanyushin, B. F. (1999) Biochemistry
(Moscow),64, 307-317.
23.Kirnos, M. D., Bakeeva, L. E., Volkova, S. A.,
Ganicheva, N. I., and Vanyushin, B. F. (1983) Biokhimiya,
48, 1505-1512.
24.Kirnos, M. D., Aleksandrushkina, N. I., Shorning,
B. Yu., Bubenshchikova, S. N., and Vanyushin, B. F. (1997)
Biochemistry (Moscow),62, 1348-1357.
25.Kirnos, M. D., Alexandrushkina, N. I.,
Zagorskaya, G. Ya., Kireev, I. I., and Vanyushin, B. F. (1992) FEBS
Lett., 298, 109-112.
26.Bolton, J. L., and Thompson, J. A. (1991) Drug
Metab. Dispos., 19, 467-472.
27.Dwyer-Nield, L. D., Thompson, J. A., Peljak, G.,
Squier, M. K., Barker, T. D., Parkinson, A., Cohen, J. J., Dinsdale,
D., and Malkinson, A. M. (1998) Toxicology, 130,
115-127.
28.Smirnova, E. G., Lyubimov, Yu. I., Malinina, T.
G., Lyubimova, E. Yu., Aleksndrushkina, N. I., Vanyushin, B. F., and
Yaguzhinsky, L. S. (2002) Biochemistry (Moscow),67, in
press.
29.Bouvier, F., Backhaus, R. A., and Camara, B.
(1998) J. Biol. Chem., 273, 30651-30659.
30.Schnurr, K., Hellwing, M., Seidemann, B.,
Jungblut, P., Kuhn, H., Rapoport, S. M., and Schewe, T. (1996) Free
Radic. Biol. Med.,20, 11-21.
31.Skulachev, V. P. (1999) Mol. Aspects Med.,
20, 139-184.