Interaction of 65- and 62-kD Proteins from the Apical Membranes of the Aedes aegypti Larvae Midgut Epithelium with Cry4B and Cry11A Endotoxins of Bacillus thuringiensis
A. A. Buzdin, L. P. Revina, L. I. Kostina, I. A. Zalunin*, and G. G. Chestukhina
Institute of Genetics and Selection of Industrial Microorganisms, 1-yi Dorozhnyi Proezd 1, Moscow, 113545 Russia; fax: (095) 315-0501; E-mail: lab_11@genetika.ru* To whom correspondence should be addressed.
Received November 19, 2001
A protein with the molecular weight of 65 kD is the only component of Aedes aegypti larvae BBM capable to specifically bind mosquitocidal toxins Cry4B and Cry11A of Bacillus thuringiensis. This protein lacks the leucine aminopeptidase activity which is characteristic for the toxin-binding proteins from the membranes of caterpillars. Cry-toxins inactive against A. aegypti larvae either fail to bind to the 65-kD protein and to a putative product of its proteolysis with the molecular weight of 62 kD (Cry1Ab), or bind but do not compete for this binding with mosquitocidal proteins (Cry9A). The proteolytic splitting out of the first five alpha-helices in the Cry4B toxin molecule does not affect its binding to the 65- and 62-kD proteins, but an additional removal of 20-30 amino acids from the C-terminal of the molecule sharply spoils this binding. Monosaccharide residues are not involved in the binding of the 65- and 62-kD proteins with Cry4B, Cry11A, and Cry9A.
KEY WORDS: delta-endotoxins, Bacillus thuringiensis, receptors, affinity chromatography
delta-Endotoxins of Bacillus thuringiensis are a numerous class of proteins killing larvae of many insects. And different toxins have different sets of sensitive insect species [1]. Thus, endotoxins of the Cry1A family affect caterpillars of Lepidoptera, whereas Cry4B and Cry11A affect larvae of Diptera [1, 2]. Many initial endotoxins are protoxins which need an activation with proteinases of the intestinal juice of insects to be converted into true toxins. Although having significantly different amino acid sequences, the true toxins have similar secondary and tertiary structures [3, 4]. Their molecules consist of three domains which are differently involved in the total toxic effect [5]. The loops connecting the secondary structure elements of the second domain are thought to be responsible for the toxin interaction with a specific toxin-binding protein exposed in the apical membrane of epithelial cells of the midgut (BBM) of sensitive insects. Such proteins are usually called receptors, although this name is somewhat incorrect because the real function of these proteins in the organism is not related with their interaction with the toxin. The binding to the receptor determines the specificity and efficiency of the toxic effect. Toxin receptors of two protein classes, aminopeptidases N [6-8] and cadherin-like proteins [9] have been recently identified in some caterpillars. Up to recent times there have been virtually no data on receptors of mosquitocidal proteins.
We have isolated earlier from BBM of Aedes aegypti proteins with molecular weights of 65 and 62 kD capable of binding with the mosquitocidal toxins Cry4B and Cry11A [10]. In the present work we continued our studies on the interaction of these proteins with toxins of B. thuringiensis.
MATERIALS AND METHODS
Isolation of entomocidal proteins. The B. thuringiensis strains were used as follows: B-2395 (ssp. israelensis), B-1167 (ssp. galleriae), and B-1225 (ssp. alesti) from the collection of the Institute of Genetics and Selection of Industrial Microorganisms (Moscow). The microorganisms were grown in a liquid medium containing 1% trypcasin (Human, Hungary), 0.2% yeast extract (Serva, Germany), and 0.6% glucose until the complete lysis of the sporangia [11]. The endotoxin crystals were isolated in the xylene-water system [11].
The Cry4B and Cry11A endotoxins were isolated from entomocidal crystals of the B-2395 strain. To prepare the Cry4B endotoxin, the crystals were treated with 0.05 M sodium carbonate buffer (pH 10.5) containing 0.01 M dithiothreitol (Serva) (buffer A) and the resulting extract was subjected to chromatography on a Mono Q column (Pharmacia, Sweden) [12, 13]. The Cry11A endotoxin not dissolved during the previous treatment of the crystals was extracted with 0.05 M NaOH [10].
To isolate the Cry9A toxin, crystals from the B-1167 strain containing a number of endotoxins were dissolved with buffer A, the resulting protoxins were activated with trypsin (Serva) at the ratio 1 : 100 (w/w) for 4 h at 37°C, and the toxin mixture was separated on a Mono Q ion exchanger [14].
The Cry1Ab toxin was prepared by dissolving crystals from the B-1225 strain with buffer A and by activation of the resulting protoxin with trypsin at the ratio 1 : 100 (w/w) for 12 h at 37°C.
Limited proteolysis of the Cry4B endotoxin. To prepare the Cry4B endotoxin fragments with molecular weights of 70, 50, and 46-48 kD, the initial protoxin was treated with chymotrypsin (Serva), trypsin, or with the intestinal juice of A. aegypti larvae, respectively, for 4 h at 37°C [13]. The enzymes were used at the ratio of 1 : 100 (w/w). To prepare the intestinal juice, the midguts isolated from 20-30 larvae were homogenized with sodium phosphate buffer (pH 7.3) containing 0.05 M NaCl and 0.001 M KCl (PBS buffer) and centrifuged for 5 min using an Eppendorf centrifuge. To 1 mg Cry4B a portion of the intestinal juice was added which contained 0.015 unit of trypsin activity that was determined with benzoyl-D,L-arginine p-nitroanilide as the substrate.
Biotinylation of proteins. The proteins were biotinylated in 0.1 M carbonate buffer (pH 9.5) containing 0.15 M NaCl by treatment with 40 µl of biotin N-succinimide ester (Amersham, England) per mg protein. The modified proteins were separated from the excess reagent by gel filtration on Sephadex G-25 (Pharmacia). The gel filtration process was followed by dot-blot analysis. A sample of every fraction (1 µl) was placed onto a nitrocellulose filter. The nonspecific binding was blocked with 1% BSA (Sigma, USA) in PBS buffer containing 0.3% Tween 20 (Serva) at room temperature. Then the filters were incubated for 1 h in PBS buffer containing streptavidin-alkaline phosphatase conjugate (Amersham) diluted 2000-fold, carefully washed, and treated with 0.1 M Tris-HCl buffer (pH 9.5) containing 0.1 M NaCl, 0.01 M MgCl2, and substrates of the phosphatase reaction (NBT/BCIP, Promega, USA).
Obtaining of BBM preparations from A. aegypti. Larvae of A. aegypti mosquitoes were grown in water using dry milk and yeast mixture as the nutriment. The insects entered the fourth larva stage and were homogenized with 17 mM Tris-HCl buffer (pH 7.5) containing 0.3 M mannitol (Sigma) and 5 mM EGTA (Sigma). BBM preparations were obtained from the homogenate by precipitation with magnesium ions combined with differential centrifugation (four cycles) [10, 15, 16]. The specific activities of the marker enzyme (leucine aminopeptidase) in the initial homogenate and in the resulting preparation (with leucine p-nitroanilide as the substrate) were compared, and BBM preparations were found to be 4.5-fold purified.
The synthesis of an affinity sorbent Cry11A-Sepharose. Solution of Cry11A (8 ml) in 0.05 M carbonate buffer (0.6 mg/ml), pH 10.0, was mixed with 5 ml of Sepharose 4B (Serva) activated with cyanogen bromide [10]. The mixture was incubated at room temperature in a shaker for 24 h. Free activated groups of the sorbent were blocked with 0.2 M glycine. The linking of the protein was analyzed with a Biotronik LC 5001 amino acid analyzer (Germany) after hydrolysis of a vacuum-dried aliquot of the sorbent with 6.0 M HCl. The resulting preparation of Cry11A-Sepharose contained 0.89 mg (12.7 nmol) protein per ml of the sorbent.
Affinity chromatography of the membrane protein extract. The extract of BBM proteins (6 mg/ml) was prepared by treatment of the membranes with 0.1 M carbonate buffer (pH 9.5) containing 1 µM EDTA, 1 µM diisopropyl fluorophosphate, and 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propane sulfonate (CHAPS, Sigma) for 30 min at room temperature, with a subsequent centrifugation for 20 min at 12,000 rpm.
The extract was placed onto a column containing 2 ml of Cry11A-Sepharose equilibrated with buffer B (0.1 M carbonate buffer, pH 9.5, 0.2% CHAPS, 1 µM EDTA, 1 µM phenylmethylsulfonyl fluoride) at the rate of 0.07 ml/min. Chromatography was performed as described earlier [10], with changes as follows: the elution was started immediately after the specimen loading, without the stage of the extract incubation with the sorbent. The column was successively washed with buffer B, with buffer B containing 1.5 M NaCl, and with 0.01 M NaOH containing 0.2% CHAPS (pH 12). The chromatography course was followed by electrophoresis in 10% gel in the presence of SDS. The gels were stained with silver using Silver stain kit reagents (Sigma).
Electrophoresis. Electrophoresis in the presence of SDS was performed by the method of Laemmli [17]. We used 10% gels and a set of preliminary stained proteins, such as BSA (74 kD), ovalbumin (50 kD), carboanhydrase (34 kD), soybean trypsin inhibitor (28 kD), and lysozyme (20 kD) as the standards.
Ligand-blotting. To identify the membrane components binding the mosquitocidal toxins, the extract of BBM proteins prepared by treatment with CHAPS, or the fractions resulting by affinity chromatography were subjected to electrophoresis by the Laemmli method with a subsequent electrotransfer onto a nitrocellulose membrane in a Novablot apparatus (LKB, Sweden) for 12 h at the current of 15 mA [10]. Nonspecific binding was prevented with 1% BSA in PBS buffer containing 0.3% Tween 20 at room temperature. The filters were incubated for 1 h with 0.05 M carbonate buffer (pH 9.5) containing 0.1% BSA, 0.3% Tween 20, and the biotinylated Cry11A toxin (10 µg/ml), and then washed with three changes of the above-mentioned buffer (each washing with 5 ml). Finally, the filters were successively treated with streptavidin-alkaline phosphatase conjugate and with substrates of the phosphatase reaction as described above.
To determine the affinity of 65- and 62-kD proteins to the toxins, the protein-containing fractions resulting by affinity chromatography were studied using electrophoresis, electrotransfer, and incubation with ligands as described above. The biotinylated toxins Cry4B, Cry11A, Cry1Ab, or Cry9A, and also fragments of the Cry4B toxin molecule at the concentration of 10 µg/ml were used as ligands.
Experiments on homological and heterological competition. The nitrocellulose filters with the 65- and 62-kD proteins transferred onto them were incubated with a biotinylated variant of one of the toxins under study immediately or in the mixture with a 20-fold excess of the same protein nonbiotinylated (the homological competition) or of a supposed competitor nonbiotinylated (the heterological competition). Then the filters were treated as described above.
Studies on the effects of monosaccharides on the binding of biotinylated proteins to receptors. The nitrocellulose filters with the 65- and 62-kD proteins transferred onto them were incubated with the biotinylated toxins in the absence or in the presence of 0.1 M glucose, galactose, galactosamine, N-acetylglucosamine, N-acetylgalactosamine, or mannose (all reagents were from Sigma) and also in the presence of monosaccharide mixtures of the following composition: 0.1 M galactosamine, 0.1 M acetylglucosamine, and 0.1 M N-acetylgalactosamine (a); 0.1 M glucose, 0.1 M galactose, and 0.1 M mannose (b). The subsequent treatment was as described above.
Studies on biological activities of the endotoxins using A. aegypti larvae. The biological testing was performed with A. aegypti larvae of the second stage. To provide the more efficient trapping by the filtering apparatus of the larvae, the Cry1Ab, Cry4B, and Cry11A toxins were precipitated by addition of 1 M citric acid to pH 4.5, incubated at this pH value for a week, then centrifuged for 10 min at 6000 rpm, and the precipitate was suspended with 0.05 M sodium citrate buffer (pH 4.5). For each suspension a series of successive 4-fold dilutions with the same buffer was prepared with concentrations from 4000 to 0.5 ng/ml. Each solution (10 ml) was transferred into plastic containers, and ten larvae were introduced. The control insects were maintained in 0.05 M sodium citrate buffer (pH 4.5). The insects were maintained at 28°C for 24 h and the percent mortality was counted. All experiments were performed in six replicates.
During the storage, the Cry9A toxin spontaneously precipitated from the concentrated solutions (more than 4 mg protein per ml of 0.05 M Tris-HCl buffer, pH 8.0). The precipitate was centrifuged and suspended in 0.05 M sodium citrate buffer (pH 4.5). The further determination of the activity was performed as described above.
Determination of the protein concentration. The protein concentration in the toxin solutions was determined by absorption at 280 nm, assuming that the A1%1cm value for solutions of entomocidal proteins was 16.
The concentration of membrane proteins was determined by the method of Bradford [18] (when working with the membrane preparations) or by the Lowry method (when working with the membrane protein extracts), using reagents and methods of Bio-Rad (USA) and with BSA as the standard.
RESULTS AND DISCUSSION
Identification of the toxin-binding protein in BBM from A. aegypti. The proteins with the molecular weight of 65- and 62-kD capable for binding to the Cry11A endotoxin and to the activated Cry4B toxin under ligand blotting conditions [10] were earlier isolated by us from A. aegypti BBM by affinity chromatography on toxin-Sepharose columns.
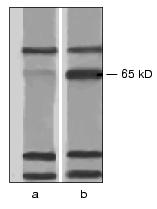
To verify the absence of other toxin-binding components in A. aegypti BBM, we have studied the interaction of the biotinylated Cry4B and Cry11A toxins with protein extracts from these membranes. Nonionic detergents (Triton X-100, beta-octyl glucoside, Nonidet P-40) and a zwitterion detergent (CHAPS) extracted from A. aegypti the same set of proteins with molecular weights from 10 to 170 kD. On interaction with these proteins under conditions of ligand blotting, the biotinylated Cry4B and Cry11A toxins easily bound to bands which corresponded to proteins with molecular weights of 70, 65, 30, and 24 kD (Fig. 1). And the bands corresponding to the 70-, 30-, and 24-kD proteins were similarly well displayed in both the experiment and control (in the latter case the nitrocellulose replica was incubated immediately with streptavidin-phosphatase omitting the stage of incubation with the toxin), and thus, seem to correspond to biotin-containing enzymes in the BBM preparation. However, the band corresponding to the 65-kD protein was not found in the control (Fig. 1). Therefore, the 65-kD protein was suggested to be the only toxin-binding component in the A. aegypti BBM and to act as a receptor of the Cry4B and Cry11A toxins in these larvae.
The protein with molecular weight of 62 kD which was eluted from the affinity sorbent together with the 65-kD protein (Fig. 2) was not detected by ligand blotting at the analysis of the BBM total protein extract (Fig. 1). Most likely, it was a product of a limited proteolysis of the 65-kD component during the purification. This was confirmed by the finding that in the fractions eluted from the affinity column the ratio of 65- and 62-kD proteins varied from experiment to experiment and by appearance at electrophoresis of minor bands located between the two main components.Fig. 1. Interaction of A. aegypti BBM proteins with the Cry11A toxin under conditions of ligand blotting. The CHAPS-extract of membrane proteins was subjected to electrophoresis with a subsequent electrotransfer onto a nitrocellulose filter. In experiment (b) the filter was successively incubated with the biotinylated Cry11A and with streptavidin-alkaline phosphatase conjugate, in the control (a) it was incubated only with the conjugate.
Receptors to larvae toxins detected by now are of two classes of membrane proteins: cadherin-like proteins (210 kD) [9] and aminopeptidases N (120-170 kD) [6-8]. Because of this significant difference in the molecular weight, it was suggested [10] that the 65-kD protein from A. aegypti membranes should be of an unknown class of endotoxin receptors. This was confirmed by a complete absence of the leucine aminopeptidase activity in the fractions containing the 65- and 62-kD proteins eluted from the affinity column under relatively mild conditions compared to the previous work (Fig. 2).Fig. 2. Affinity chromatography of A. aegypti BBMproteins. The chromatography of the CHAPS-extracted membrane proteins was performed on a column with Cry11A-Sepharose. Aliquots (10 µl) were taken from the “slip” (S) and from the fractions eluted with the equilibrating buffer (B1-B4), with 1 M NaCl (s1-s9), and with 0.01 M NaOH (pH 12) (alk2-alk10) and subjected to electrophoresis in the presence of SDS. The gels were stained using a Silver stain kit. M, marker proteins, to the left their molecular weights in kD are shown.
It seems that the proteins of known classes of the Cry-toxin receptors because of their structure or location in the membranes fail to promote the toxin bound to effectively produce ion channels in membranes of the intestinal epithelium of mosquito larvae. Thus, this was suggested to be due to significant difference in the anchoring in the membrane of aminopeptidases N from Diptera and Lepidoptera larvae. It was suggested that during evolution mosquitocidal toxins should adapt for the binding to membrane proteins of another class more suitable for providing the effective interaction of the toxins with BBM of Diptera.
Interaction of 65- and 62-kD proteins with toxins active against Lepidoptera larvae. We have shown in the previous work [10] the competition of Cry4B and Cry11A for the binding to 65- and 62-kD proteins. This indicated that both toxins were binding to the same site of the receptor or to adjacent sites. It was also interesting to study the interaction of the isolated proteins with toxins of another specificity.
The biological testing on A. aegypti larvae has shown that two toxins highly effective against some caterpillar species, such as Cry1Ab from the alesti subspecies and Cry9A from the galleriae subspecies, were not toxic for A. aegypti larvae even at the concentration of 4000 ng/ml. For comparison, the LC50 values for the mosquitocidal toxins Cry4B and Cry11A from the A. aegypti strain used were 0.2 and 2 ng/ml, respectively.
In the experiments with ligand blotting, the biotinylated toxin Cry1Ab failed to bind to 65- and 62-kD proteins (Fig. 3). This seems to be because these proteins (judged from their molecular weights) are not cadherin-like proteins which are Cry1Ab receptors in caterpillars [9].
In its turn, the biotinylated Cry9A toxin was easily binding to the supposed receptor of the Cry11A and Cry4B toxins (Fig. 3). However, the Cry9A was binding to another site of these proteins than the mosquitocidal toxins. And really, in the experiments with the heterological competition an excess of the non-biotinylated Cry11A toxin completely prevented the binding of the biotinylated Cry4B and vice versa (Fig. 4), whereas no competition for the binding to 65- and 62-kD proteins was found in the toxin pairs Cry9A/Cry11A and Cry9A/Cry4B, no matter what protein was taken as the biotinylated derivative. Figure 3 shows this for the pair Cry9A/Cry11A.Fig. 3. Interaction of 65- and 62-kD proteins from A. aegypti BBM with toxins active against Lepidoptera larvae. These proteins were purified by affinity chromatography and subjected to electrophoresis with the subsequent electrotransfer onto nitrocellulose filters. The filters were incubated with the following toxins: the biotinylated Cry9A (a, c1), the biotinylated Cry9A in the presence of a 20-fold excess of non-biotinylated Cry11A (c2), the biotinylated Cry1Ab (b). Then the filters were successively treated with streptavidin-alkaline phosphatase conjugate and with substrates of the phosphatase reaction.
The Cry9A toxin was earlier shown to bind to the 60-kD protein from the midgut of an insect Galleria mellonella, which was sensitive to it (unpublished data). G. mellonella is somewhat different from most caterpillars of other species in histology of the midgut [19], and this seems to be responsible for its resistance to the majority of Cry-toxins known. It was suggested that to successfully affect BBM of this larva, the Cry9A toxin had to adjust itself during evolution to fit a representative of the same class of the Cry-toxin receptors with which Cry11A and Cry4B could interact. According to Bravo [20], molecules of Cry9A and of mosquitocidal proteins have some similarity just in the second domain which is responsible for the binding to the receptor. It was said above that the Cry9A was binding to another site of the supposed receptor compared to the binding of the mosquitocidal toxins. The absence of the Cry9A toxic effect on A. aegypti larvae suggests that in this case the toxin binding in this site is ineffective for mosquitoes.Fig. 4. Homological and heterological competitions of the biotinylated Cry4B and Cry11A toxins for the binding to 65- and 62-kD proteins from A. aegypti BBM. These proteins were purified by affinity chromatography and subjected to electrophoresis with a subsequent electrotransfer onto nitrocellulose filters. The filters were incubated with the following toxins: the biotinylated Cry4B (a1, b1), the mixture of the biotinylated Cry4B with a 20-fold excess of non-biotinylated Cry4B (a2) or Cry11A (b2), the biotinylated Cry11A (c1, d1), the mixture of the biotinylated Cry11A with a 20-fold excess of non-biotinylated Cry11A (c2) or Cry4B (d2). Then the filters were treated as described in the Fig. 3 caption.
Studies on the roles of separate elements of the Cry4B toxin structure in the binding to 65- and 62-kD proteins. The mosquitocidal toxins have a clearly pronounced domain organization [21]. To elucidate what regions of the mosquitocidal toxin molecule are responsible for the binding to 65- and 62-kD proteins, we used products of a limited proteolysis of the Cry4B endotoxin. The course of the limited proteolysis of this protein depended on the enzyme chosen [13]. Under the influence of chymotrypsin a true toxin was produced with the molecular weight of 70 kD, trypsin split out from the true toxin the first five alpha-helices of the N-terminal domain (the 50-kD fragment), and proteinases of the intestinal juice of A. aegypti larvae additionally split out 20-30 residues from the C-terminal of the molecule (the 46- and 48-kD fragments).
Figure 5 shows that under conditions of ligand blotting, the 50-kD fragment binds to 65- and 62-kD proteins with similar efficiency as the 70-kD toxin. Thus, the first five alpha-helices are not required for the binding, and this corresponds to the current concepts that the role of the N-terminal domain in the toxin effect is limited to the membrane perforation.
The 46- and 48-kD fragments, in their turns, were binding to the 65- and 62-kD proteins very poorly (Fig. 5). It was suggested that the C-terminal fragment of the Cry4B toxin which corresponded to two-three elements of the beta-structure of the third domain of the molecule [4, 5] should be involved in the binding to the receptor either directly or through the formation of a necessary tertiary structure of the whole molecule. The latter suggestion is partially confirmed by the finding that in the region split out the fifth conservative block of the primary structure of the Cry-proteins occurred, which formed the central beta-leaf of the domain 3 and was also involved in the formation of several intramolecular bridges [3]. The worsening of the binding to the receptor of the 46- and 48-kD fragments of the Cry4B molecule correlated with a 30-fold decrease in the toxicity for A. aegypti [13]. Moreover, according to data of [22], the fragments with molecular weights of 46 and 48 kD acquired the toxicity for certain mosquito non-epithelial cell lines for which the 50- and 70-kD fragments were nontoxic. Obviously, in this case the destabilization of the third domain of the molecule not only prevented the interaction with the 65- and 62-kD proteins but also promoted the conformation that made easier the interaction with some new receptors.Fig. 5. Interaction of 65- and 62-kD proteins from A. aegypti BBMwith products of the limited proteolysis of the Cry4B endotoxin. The filters containing 65- and 62-kD proteins from A. aegypti BBMwere incubated with biotinylated products of the Cry4B limited proteolysis with molecular weights as follows: 70 kD (the true toxin (a)), 46- and 48-kD (b), and 50 kD (c). Then the filters were treated as described in the Fig. 3 legend.
Studies on a putative involvement of carbohydrate residues in the interaction of mosquitocidal toxins with the 65- and 62-kD proteins of A. aegypti. It was suggested [23] that mosquitocidal toxins produced by israelensis ssp. should be glycoproteins and that their carbohydrate residues should be involved in the binding to the receptor.
However, we have shown that under conditions of ligand blotting, the binding of the Cry4B, Cry11A, or Cry9A toxins to the 65- and 62-kD proteins was not decreased on addition into the incubation medium of mannose, glucose, galactose, galactosamine, N-acetylglucosamine, or N-acetylgalactosamine and also of their mixture. The absence of the N-galactosamine effect on the binding of the Cry4B and Cry11A toxins to the 65- and 62-kD proteins is illustrated by Fig. 6. The findings suggested that in these cases the toxin interacted with the receptor without the involvement of carbohydrate components which could be present in the interacting proteins. This was also confirmed by failure of attempts to elute the 65- and 62-kD proteins from Cry4B- and Cry11A-Sepharose with 0.1 M N-acetylglucosamine or N-acetylgalactosamine.
The data obtained are fundamentally different from the findings [24] for the case of the Cry1Ac interaction with aminopeptidase N when N-acetylgalactosamine residues in the receptor are involved in the binding of the toxin.Fig. 6. Effect of N-acetylgalactosamine on the interaction of 65- and 62-kD proteins from A. aegypti BBM with the Cry4B and Cry11A toxins. The filters containing 65- and 62-kD proteins from A. aegypti BBM were treated with the biotinylated Cry4B and Cry11A toxins in the absence (a1, b1) and in the presence of 0.1 M N-acetylgalactosamine (a2, b2). Then the filters were treated as described in the Fig. 3 legend.
The experimental results have confirmed the earlier suggestion that the 65-kD protein should be a receptor of mosquitocidal toxins in the intestinal epithelium of mosquito larvae, but further studies are required to prove this finally.
REFERENCES
1.Hofte, H., and Whiteley, H. (1989) Microbiol.
Rev., 53, 242-255.
2.Crickmore, N., Zeigler, D. R., Feitelson, J.,
Schnepf, E., van Rie, J., Lereclus, D., Baum, J., and Dean, D. H.
(1998) Microbiol. Mol. Biol. Rev., 62, 807-813.
3.Grochulski, P., Masson, L., Borisova, S.,
Pusztai-Carey, M., Schwartz, J.-L., Brousseau, R., and Cygler, M.
(1995) J. Mol. Biol., 254, 447-464.
4.Hodgman, T. C., and Ellar, D. G. (1990) J. DNA
Sequensis, 1, 97-106.
5.Li, J., Carrol, J., and Ellar, D. J. (1991)
Nature, 353, 815-821.
6.Gill, S. S., Cowles, E. A., and Francis, V. (1995)
J. Biol. Chem., 270, 27277-27282.
7.Knight, P. J. K., Crickmore, N., and Ellar, D. J.
(1994) Mol. Microbiol., 11, 429-436.
8.Valaitis, A. P., Lee, M. K., Rajamohan, F., and
Dean, D. H. (1995) Insect Biochem. Mol. Biol., 25,
1143-1151.
9.Valdamudi, R. K., Weber, E., Ji, I., Ji, T. H., and
Bulla, L. (1995) J. Biol. Chem., 270, 5490-5494.
10.Krieger, I. V., Revina, L. P., Kostina, L. I.,
Buzdin, A. A., Zalunin, I. A., Chestukhina, G. G., and Stepanov, V. M.
(1999) Biochemistry (Moscow), 64, 1163-1268.
11.Chestukhina, G. G., Kostina, L. I., Zalunin, I.
A., Kotova, T. S., Katrukha, S. P., Kuznetsov, Yu. S., and Stepanov, V.
M. (1977) Biokhimiya, 42, 1660-1667.
12.Chestukhina, G. G., Zalunin, I. A., Kostina, L.
I., Bormatova, M. E., Klepikova, F. S., Khodova, O. M., and Stepanov,
V. M. (1985) FEBS Lett., 190, 345-348.
13.Zalunin, I. A., Revina, L. P., Kostina, L. I.,
Chestukhina, G. G., and Stepanov, V. M. (1998) J. Prot. Chem.,
17, 463-471.
14.Chestukhina, G. G., Kostina, L. I., Zalunin, I.
A., Revina, L. P., Mikhailova, A. L., and Stepanov, V. M. (1994)
Can. J. Microbiol., 40, 1026-1034.
15.MacIntosh, S. C., Lidster, B. D., and Kirkham, C.
L. (1994) J. Invertebrate Pathol., 63, 97-98.
16.Wolfersberger, M., Luethy, P., Maurer, A.,
Parenti, P., Sacchi, F. V., Giordana, B., and Hanozet, G. M. (1987)
Comp. Biochem. Physiol., 86A, 301-308.
17.Laemmli, U. K. (1970) Nature, 227,
680-685.
18.Bradford, M. M. (1976) Analyt. Biochem.,
72, 248-254.
19.Hoopingarner, R., and Materu, M. E. A. (1964)
J. Insect Pathol., 6, 26-30.
20.Bravo, A. (1997) J. Bacteriol.,
179, 2793-2801.
21.Chestukhina, G. G., Tyurin, S. A., Kostina, L.
I., Osterman, A. L., Zalunin, I. A., Khodova, O. A., and Stepanov, V.
M. (1990) J. Prot. Chem., 9, 501-507.
22.Angsuthanasombat, C., Crickmore, N., and Ellar,
D. J. (1993) FEMS Microbiol. Lett., 111, 255-262.
23.Muthukumar, G., and Nickerson, K. W. (1987)
Appl. Environ. Microbiol., 53, 2650-2655.
24.Knowles, B. H., Knight, P. J. V., and Ellar, D.
J. (1991) Proc. Roy. Soc. Lnd, B., 245, 31-35.