Dependence of PrePhoA-Phospholipid Interaction in vivo and in vitro on Charge of Signal Peptide N-Terminus and Content of Anionic Phospholipids in Membranes
S. N. Zolov, N. I. Mikhaleva, A. E. Kalinin, and M. A. Nesmeyanova*
Laboratory of Protein Secretion in Bacteria, Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, pr. Nauki 5, Pushchino, Moscow Region, 142290 Russia; fax: (095) 956-3370; E-mail: aniram@ibpm.serpukhov.su* To whom correspondence should be addressed.
Received March 27, 2002; Revision received April 3, 2002
Replacement of the positively charged signal peptide with neutral or negatively charged peptides due to substitution of Lys(-20) in the N-terminal region of the signal peptide leads to decreases in the rate of prePhoA membrane translocation in vivo and in the efficiency of prePhoA insertion into liposomes in vitro. The effect of anionic phospholipids on prePhoA insertion into model membranes is determined by the signal peptide N-terminus charge, while the dependence of prePhoA translocation across the cytoplasmic membrane in vivo is not, under the studied variations in the content of anionic phospholipids. This is evidence of the possibility of direct electrostatic interaction between the signal peptide N-terminus and anionic phospholipids, which in vivo, however, seems to involve some proteins of the Sec machinery.
KEY WORDS: Escherichia coli, alkaline phosphatase, signal peptide, amino acid substitutions, protein translocation, anionic phospholipids, liposomes
Abbreviations: PhoA) mature form of alkaline phosphatase of Escherichia coli; prePhoA) precursor of the alkaline phosphatase; APL) anionic phospholipids; PA) phosphatidic acid; PG) phosphatidylglycerol; CL) cardiolipin; PE) phosphatidylethanolamine; IPTG) isopropyl-1-thio-beta-D-galactopyranoside; SDS-PAGE) sodium dodecyl sulfate-polyacrylamide gel electrophoresis; EDTA) ethylenediaminetetraacetic acid; TCA) trichloroacetic acid; PIPES) piperazine-N,N-bis-2-ethanesulfonic acid; DTT) dithiothreitol; DPH) 1,6-diphenyl-1,3,5-hexatriene.
The proteins of Escherichia coli destined to be secreted into
periplasm or outer membrane are synthesized as precursors containing
additional 18-30 amino acid residues at the polypeptide chain
N-terminus--the signal peptide (SP). The SP is absolutely necessary for
protein recognition by the cell secretory machinery and for initiation
of protein translocation across the cytoplasmic membrane [1, 2]. SPs of all secretory
proteins of E. coli possess at least one positively charged
amino acid in their N-termini [3]. Previously we
suggested [4, 5] that an
important role in secretion is played by protein-lipid interactions
determined by the positively charged signal peptide N-terminus and
negatively charged anionic phospholipids (APL) of membranes. At
present, the necessity of the N-terminal positive charge for protein
translocation [6-12] and
involvement of anionic phospholipids in this process [13-16] has actually been
demonstrated. However, most translocation reactions used the outer
membrane proteins [6, 8, 10, 11, 13-15, 17-19], while the data on
soluble secreted (periplasmic) proteins are very limited [12, 20, 21]. In our previous works [12,
22] we obtained evidence of direct interaction of
anionic phospholipids with the positively charged N-terminus of prePhoA
signal peptide in the in vivo system. It was shown that the
blockage of processing due to amino acid substitutions in the site of
SP cleavage by signal peptidase leads to prePhoA “anchoring”
in the cytoplasmic membrane (signal peptide in the
Nin-Cout orientation) and simultaneous
accumulation of both the precursor and anionic phospholipids in the
membrane [21]. An additional replacement of a
positively charged Lys(-20) by negatively charged Glu or uncharged Ala
in mutant proteins with uncleaved SP prevents the accumulation of APL
even at the retention of anchored prePhoA in the membrane [12]. However, anionic phospholipids were shown to be
involved in protein translocation at different stages, intensifying the
function of protein components of the secretory machinery in the in
vivo system [14, 16, 23, 24]. So, anionic
phospholipids contribute to membrane binding, membrane insertion [25], and conformational changes [26] of the SecA protein--one of the essential
components of the secretory machinery [27, 28], providing the maximal functioning of this
protein [16]. Thus, the assessment of dependence
of protein translocation on anionic phospholipids at the molecular
level is complicated by the necessity of these phospholipids for the
function of protein components of the secretory machinery. Questions
arise, what is the mechanism of involvement of the N-terminal charged
region of the SP and anionic phospholipids in protein translocation
across the cytoplasmic membrane and is there a direct interaction
between them, or some protein components of the secretory machinery
determine the dependence of protein translocation on the positive
charge of the SP N-terminus and anionic phospholipids?
The main strategy addressing the above questions in the current work is using simultaneously the wild-type and mutant prePhoAs possessing different charges of the SP N-terminus, on one hand [22], and Escherichia coli strains with a controlled content of anionic phospholipids [15, 23], on the other hand. The in vivo and an in vitro systems were used as well, in particular liposomes derived from phospholipids isolated from these strains and containing no protein factors [20], as well as isolated prePhoA [22]. The prePhoA interaction with liposomes in vitro was shown determined by the signal peptide charge depending on the anionic phospholipid content in liposomes, which indicates a direct electrostatic interaction of the signal peptide N-terminus with anionic phospholipids, while this interaction in vivo was proved to depend on the Sec machinery.
MATERIALS AND METHODS
Bacterial strains and plasmids. E. coli strain E15 (Hfr DeltaphoA8 fadL701 tonA22 garB10 ompF627 relA1 pit-10 spoT1 T2) [29] was used as a host strain for the expression of the wild-type and mutant alkaline phosphatase genes (phoA) and for prePhoA isolation. Cells of E. coli strains HDL11 [15] and AD93 [30] with the controlled synthesis of anionic phospholipids and zwitterionic phospholipid, respectively, were used to obtain lipids for preparation of model membranes (liposomes) with different contents of anionic phospholipids. Phagemid pPHOA12 [31, 32] with the wild-type phoA gene was used to construct mutant phoA genes [22]. Plasmids pSAP-1, pSAP-2, and pSAP-3, where the wild-type and mutant phoA genes are under the control of PBAD promoter of the arabinose operon (araBAD) constituent of plasmid pBAD18 [33], have been constructed in this work to change the regulation of phoA genes expression. E. coli strain Z85 (thi Delta(lac-proAB) Delta(srl-recA) hsdR::Tn10 (F´ traD proAB lacIq lacZDeltaM15)) [34] was used in experiments on gene cloning.
Media and conditions of cultivation. Bacteria for gene cloning were grown on LB or 2YT media at 37°C [35]. Strain E. coli AD93, which requires divalent cations for growth, was grown in the presence of MgCl2 (50 mM) and tryptophan (100 µg/ml) [30]. Cells of E. coli HDL11 were grown in the presence of isopropyl-1-thio-beta-D-galactopyranoside (IPTG) (100 µM) [15] to induce the synthesis of anionic phospholipids. Kanamycin (50 µg/ml), tetracycline (14 µg/ml), ampicillin (100 µg/ml), and chloramphenicol (25 µg/ml) were added to the growth medium as required. Secretion of wild type and mutant PhoA was studied in cells grown to the mid-log phase on a mineral medium [36] supplemented with 0.07% peptone, 0.5% glucose, and 1 mM orthophosphate. For derepression of PhoA synthesis, the cells were separated from culture liquid by centrifugation (14,000g at 37°C), washed with 0.14 M NaCl at 37°C, and resuspended in the same medium without peptone but containing 0.5% arabinose. The cells were incubated for 1 h at 37°C. Biosynthetic processes were stopped by merthiolate at final concentration 0.02%.
Plasmid construction. Isolation of plasmid DNA, electrophoresis of DNA fragments in agarose gel, and transformation of E. coli cells were carried out according to the protocols of Sambrook et al. [37]. The nucleotide sequence of DNA was determined using a modified DNA-polymerase of phage T7 by the method of Sanger et al. [38]. Mutant alleles of the phoA gene were obtained using modified oligonucleotide-directed mutagenesis by the protocols of Promega (USA) [22], which is a combination of classical oligonucleotide-directed mutagenesis, amber-suppressor mutagenesis, and PhoA phenotype color test. To clone the phoA genes under the control of arabinose PBAD promoter, the wild-type and mutant phoA genes were amplified by PCR from phagemid pPHOA12 [31] using the following pair of primers: (5´-AATGCTCGAGTACATGGAGAAAATAAAGTG-3´) and (5´-ATCTGGATCCAAGTCTGGTTGCTAACAGC-3´), with the sites of restriction endonucleases XhoI and BamHI introduced in their sequences, respectively. PCR products were cloned by the XhoI and BamHI sites in the vector pBC (Stratagene, USA). Then these genes were cut out by the KpnI-XbaI sites and cloned in the vector pBAD18 [33]. The mutations were confirmed by DNA sequencing.
Maturation of alkaline phosphatase. Maturation of prePhoA was analyzed by pulse labeling of proteins in vivo. The synthesis of PhoA, whose gene was under the control of PBAD promoter, was induced for 10 min by addition of 0.5% arabinose to the cells of E. coli grown to the mid-log phase. The proteins were pulse labeled for 30 sec with the addition of L-[35S]methionine to the medium (50 µCi/ml). Incorporation was stopped by addition of unlabeled amino acid to the final concentration 0.05%. Culture samples were taken in certain periods of time, proteins were precipitated by 10% TCA, prePhoA and PhoA were immunoprecipitated [39] using rabbit antibodies against denatured alkaline phosphatase and separated by electrophoresis followed by autoradiography. Densitometry of autoradiographs was made on an UltroScan laser densitometer (Pharmacia LKB, Sweden) and the peak area was used to calculate the relative amounts of the precursor and the mature enzyme form.
Lipid analysis. Lipids from cells of different E. coli strains were extracted as described [40] with a mixture of chloroform-methanol-water. Thin layer chromatography of the phospholipids was carried out on silica gel Kieselgel 60 (Merck, Germany) plates (impregnated with 1.2% boric acid in 50% ethanol) in the system of chloroform-methanol-water-34% ammonium hydroxide (60 : 37.5 : 3 : 1 v/v) [41]. Individual lipid spots were detected by iodine vapor, cut out and extracted from silica gel with chloroform-methanol-water (5 : 5 : 1 v/v) and the content of lipid phosphorus was measured as described previously [42].
Liposomes and prePhoA. Dry lipid preparation was suspended in buffer containing 100 mM NaCl, 10 mM PIPES, 2 mM EDTA, pH 7.5 (lipid phosphorus concentration was 5 mM), and the mixture was subjected to 10 cycles of ultrasonic treatment for 30 sec (50 W) at 4°C using an MSE ultrasound disintegrator (England). Large multilamellar structures were removed by centrifugation at 30,000g for 10 min at 4°C. The supernatant, which has been shown [43] to contain small unilamellar vesicles of 100-200 nm in diameter, was used at a lipid concentration of 25-50 µM. Wild-type and mutant prePhoAs were isolated previously [22].
Fluorescent spectroscopy. PrePhoA interaction with liposomes was estimated by an increase in fluorescence anisotropy of the hydrophobic probe 1,6-diphenyl-1,3,5-hexatriene (DPH) in liposomes as described previously [20]. Fluorometric measurements were made on a Hitachi 850 spectrofluorimeter (Japan) with a thermostatted cuvette-holder in quartz cuvettes (10 × 10 mm). DPH (0.1% solution in dimethylsulfoxide) in the molar ratio of probe/lipid 1 : 250 was introduced into a cuvette with 5 mM Tris-HCl, pH 7.5, and 25-50 µM lipids. After reaching equilibrium, the mixture was supplemented by the prePhoA in the molar ratio of protein/lipid 1 : 20. Fluorescence anisotropy of the probe was measured at lambdaex = 360 nm and lambdaem = 430 nm, temperature 37°C, and slit width 5 nm. The value of anisotropy r was measured by the formula: r = (I|| - I.)/(I|| + 2I.), where III and I. are fluorescence intensities with the parallel and perpendicular polarizer and analyzer, respectively.
Analytical methods. Protein electrophoresis was performed in 10% SDS-PAGE according to Laemmli [44]. Protein was assayed by the Lowry method [45]. The PhoA activity was determined by the rate of p-nitrophenylphosphate hydrolysis in 50 mM Tris-HCl buffer, pH 8.5, containing 5 mM MgCl2 [36], taking the amount of enzyme hydrolyzing 1 µmol of substrate per 1 min at 37°C as a unit of enzymatic activity (U).
RESULTS
Dependence of prePhoA translocation in vivo on anionic phospholipids is irrespective of signal peptide N-terminus charge. The translocation of wild-type and mutant PhoAs expressed from the PBAD promoter was studied. The scheme of the PhoAs signal peptides with different N-terminal charge is given in Fig. 1. The effect of anionic phospholipids (APL) on this process was analyzed in the strain E. coli HDL11, where their content is regulated by the presence of IPTG in the medium [15]. The content of APL (phosphatidylglycerol (PG) and cardiolipin (CL)) in cells of this strain, grown in a rich medium to a stationary phase is 6% in the absence and 20-25% in the presence of IPTG. We detected approximately the same content of PG plus CL in the cells grown on a mineral medium. In the cells grown to the mid-log phase, the content of PG and CL was 1.7% in the absence and 14.6% in the presence of IPTG. In the absence of IPTG we also found the higher content (up to 4.7%) of phosphatidic acid (PA), the head of which was negatively charged as well (Table 1).
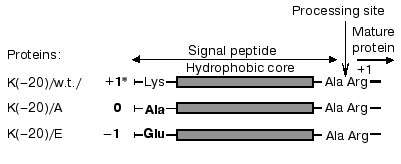
Table 1. Phospholipid composition of E. coli strains depending on cell growth conditionsFig. 1. Structure of the signal peptide (SP) of mature wild-type and mutant prePhoAs. * The charge of SP N-terminal domain. Amino acid substitutions are in bold.
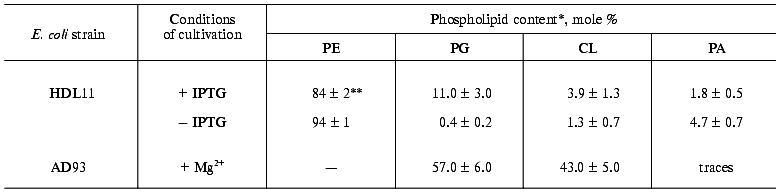
*Phospholipids were extracted from the cells grown to mid-log phase.
**Data are mean values of three independent experiments.
The efficiency of prePhoA translocation in vivo was studied by two independent approaches. Alkaline phosphatase is known to become enzymatically active only after translocation of the polypeptide chain across the cytoplasmic membrane into the periplasm, where the appropriate disulfide bonds are formed, dimerization of monomers occurs, and active macromolecules of the enzyme are formed [46, 47]. Therefore, the efficiency of prePhoA translocation in vivo can be judged by PhoA activity in cells. The other approach involved the processing/maturation assay [48]. Since the signal peptide is cleaved during or immediately after the translocation is completed [6], translocation efficiency can be estimated from the rate of conversion of prePhoA to mature PhoA. Accumulation of prePhoA would reflect either a translocation block or a decrease in translocation efficiency.
The cells ofE. coli HDL11,secreting PhoAs (both wild type and mutant) with difference in the signal peptide N-terminus charges showed a high phosphatase activity (Table 2). This indicates that the amino acid substitutions for Lys(-20) do not prohibit enzyme translocation across the cytoplasmic membrane. However, a decrease in activity of the cells secreting mutant PhoAs was observed. This decrease was independent of IPTG, indicating that the positive charge of the signal peptide is important for efficient prePhoA translocation in vivo irrespective of the APL content. Figure 2 supports the above conclusion. Pulse-labeled wild type prePhoA (K(-20)) demonstrates a rapid translocation across the cytoplasmic membrane, which is evident from a nearly complete maturation of the protein within 1 min. In contrast, prePhoA with positively charged Lys(-20) being replaced by either the neutral Ala or negatively charged Glu maturated more slowly in the same period. Thus, the results demonstrate that the positively charged N-terminus of prePhoA significantly enhances the translocation process but is not absolutely required, as it has been reported for many other secreted proteins [6, 7, 9, 48-50].
Table 2. Activity of wild-type and mutant
alkaline phosphatases in cells of E. coli HDL11 depending on the
content of anionic phospholipids
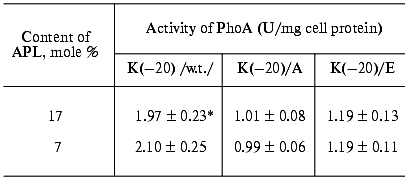
*Data are the mean values of fives independent experiments
and show activity of PhoA after 60-min induction of its
biosynthesis.
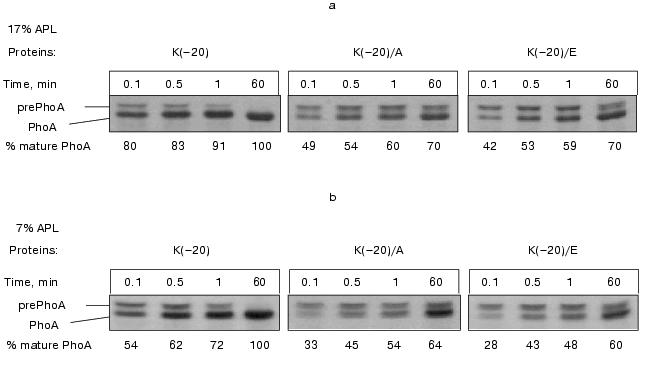
The effect of low APL content was less profound in comparison with the effect of alteration of the signal peptide N-terminus charge. In essence, no significant difference in the PhoA activity depending on IPTG was found in the cells secreting either wild type or mutant PhoAs (Table 2). However, a certain correlation between the APL content and the rate of prePhoA translocation was obtained in pulse-chase experiments. Wild type prePhoA was more rapidly converted into a mature PhoA when the APL content was high (17%) (Fig. 2a) relative to the much slower rate of maturation in cells with reduced APL content (7%) (Fig. 2b). In the first seconds of chase, a 1.5-fold lower rate of PhoA maturation was detected in such cells. However, the rate of maturation of mutant prePhoAs, which was slower than for the wild-type protein, was also positively dependent on the APL content. This indicates that the positive charge of the signal peptide is important for efficient prePhoA translocation in vivo irrespective of APL content and dependence of protein translocation on APL is not determined by the charge of the signal peptide N-terminus, at least in the range of variation of their content examined in the current work.Fig. 2. Effect of signal peptide N-terminus charge and anionic phospholipids content on prePhoA maturation in vivo. E. coli HDL11 cells producing wild type or mutant PhoAs were grown to the mid-log growth phase on a mineral medium in the presence of IPTG (a) and in the absence of IPTG (b). In 10 min after the induction of PhoA synthesis, the cells were pulse-labeled with L-[35S]methionine and radioactivity was chased for 0.1, 0.5, 1, and 60 min as described in “Materials and Methods”.
Dependence of prePhoA interaction with model membranes (liposomes) on the content of anionic phospholipids is determined by the signal peptide N-terminus charge. Despite the large scope of biophysical data on phospholipid interactions with synthetic signal peptides, the interactions between intact protein precursors and phospholipids have received much less attention [20, 22]. In the current work, liposomes with different APL contents were made from phospholipids, isolated from cells of the strain E. coli HDL11 [15] grown in the presence and absence of IPTG, and of the strain AD93 [30] unable to synthesize PE due to a lack of the phosphatidylserine synthase gene. This strain produces only PG and CL as the major membrane phospholipids, in a nearly equimolar ratio.
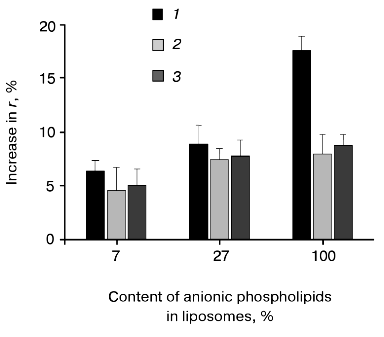
Hydrophobic fluorescence probe DPH was used to analyze the prePhoA-phospholipid interaction. This probe easily incorporates into and rapidly diffuses within the nonpolar membrane interior with almost no disturbance of the bilayer. Deep insertion of a protein into the lipid bilayer induces some restriction of the motion of acyl chains and consequently increases the DPH fluorescent anisotropy (r), which is determined by rotational diffusion of the fluorophore [51]. Indeed, the addition of wild type prePhoA to liposomes doped with DPH leads to an increase in the DPH fluorescence anisotropy, indicating the insertion of prePhoA into the hydrophobic interior of the bilayer. We have shown previously that the interaction of wild type prePhoA with liposomes containing only anionic phospholipids (derived from the strain E. coli AD93) induces a 19 and 70% increase of the fluorescence anisotropy of DPH at pH 7.0 and 4.3, respectively [20]. This pH-dependence probably reflects the involvement of ionic forces in the protein-lipid interaction. Figure 3 shows that the interaction of wild type prePhoA with liposomes is significantly enhanced as the content of anionic phospholipid increases. The interaction with liposomes consisting of anionic phospholipids only is twice as efficient as with liposomes containing the wild type level of APL (27%) and three times as efficient as with liposomes having the low APL content (7%).
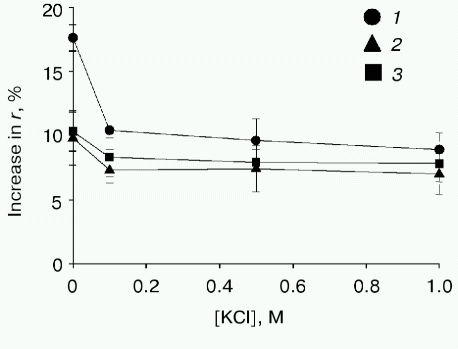
These results support the idea that electrostatic interaction between the positively charged signal peptide N-terminus and anionic phospholipid head groups promotes the insertion of prePhoA into the hydrophobic phase of the lipid bilayer. However, it is important to rule out whether the effects observed are due to the changes in lipid packing, fluidity, etc., that may occur as the phospholipid composition is altered. Therefore, the mutant prePhoAs with no positive charge in the signal peptide N-terminus were studied. Figure 3 shows that the dependence of interaction of such mutant prePhoAs with liposomes on the signal peptide N-terminus was actually less obvious at a low APL content. Additional support for electrostatic interaction between the signal peptide N-terminus and anionic phospholipids was obtained from the studies of prePhoA-phospholipid interaction in the presence of increasing amounts of KCl (Fig. 4). Even 0.1 M KCl significantly decreases the efficiency of subsequent interaction of all prePhoAs with liposomes. Besides, the degree of inhibition of the wild-type protein interaction was higher (1.7-fold) than that of mutant proteins (1.3-fold). Thus, prePhoA-phospholipid interaction in vitro, as compared with the in vivo system, is determined by the signal peptide charge depending on the content of anionic phospholipids. This confirms a possibility of the direct electrostatic interaction between the signal peptide and APL. Probably, in vivo such interaction is prevented or is not revealed due to the involvement of some proteins of the secretory machinery, e.g., SecA, in this process.Fig. 3. Interaction of PrePhoA with model membranes in vitro, depending on the signal peptide N-terminus charge and anionic phospholipid content. Insertion ofwild-type prePhoA (1) or mutant (K(-20)/A (2) or K(-20)/E (3)) prePhoA into liposomes with different contents of anionic phospholipids. The increase in DPH fluorescence anisotropy (r) was calculated from its steady-state in liposomes before prePhoA injection.
It should also be noted that the increase in the APL content in liposomes correlates with the decrease in the initial fluorescence anisotropy of DPH: 0.190, 0.186, and 0.176 at the anionic phospholipid contents 6, 27, and 100%, respectively. This points to the higher mobility of the probe and consequently to the higher “fluidity” of the lipid bilayer on increasing the content of anionic phospholipids. This is not surprising, because unsaturated fatty acids are known to be predominant in anionic phospholipids [52]. Previously we found a positive correlation between the increase in membrane “fluidity” and the efficiency of PhoA secretion in vivo [53, 54]. It is quite probable that membranes with the higher “fluidity” (low lateral pressure in the region of acyl chains of phospholipids) are more competent for protein insertion into the lipid bilayer interior, which may also contribute to the positive effect of anionic phospholipids on translocation of secreted protein precursors.Fig. 4. Effect of KCl on the prePhoA-phospholipid interaction in vitro. The interaction of wild type prePhoA (1) or mutant prePhoAs (K(-20)/A (2) and K(-20)/E (3)) with liposomes from lipids of E. coli AD93 cells containing 100% anionic phospholipids. KCl was added to liposomes before the addition of protein. The increase in DPH fluorescence anisotropy (r) was calculated from its steady-state in liposomes before prePhoA injection.
DISCUSSION
Previously we suggested an important role in secretion initiation for protein-lipid interactions determined by positively charged residues of the signal peptide and negatively charged residues of anionic phospholipids [4, 5]. The signal peptide of prePhoA has all features of the canonic signal peptide. It contains an N-terminal region, positively charged due to the presence of Lys(-20), followed by a hydrophobic core, and then a C-terminal region with the processing site. In contrast to most other secreted proteins, prePhoA also contains a positively charged residue in the C-terminus of the signal peptide (Lys(-2)) and in the N-terminus of mature moiety of the protein (Arg(+1)), which may be involved in the interaction with anionic phospholipids at initial steps of membrane protein translocation. However, we have demonstrated that substitutions of different amino acids for Arg(+1) or Lys(-2) [55] had no pronounced effect on the translocation process, and only the substitutions of uncharged or negatively charged residues for Lys(-20) hampered prePhoA translocation [12]as shown by the dynamics of maturation of mutant prePhoAs. In essence, the same data were obtained from in vivo experiments with prePhoE, where two Lys residues at the N-terminus of the signal peptide were also replaced by negative or uncharged residues [6]. These positively charged residues were shown not essential for protein translocation per se, but significantly increased its efficiency. Disturbance of the translocation could be explained by a reduced rate of initial interaction of preproteins with the cytoplasmic membrane. However, there arises a question about the kind of interaction--whether it is the interaction of the positively charged signal sequence N-terminus with anionic phospholipids [4, 12] or it involves protein components of the secretory machinery [14, 16].
We recently demonstrated that Lys(-20) of prePhoA could be involved in the direct interaction of SP with membrane anionic phospholipids in vivo [22]. In the current work we have extended these studies and demonstrated using new and novel approaches that the substitutions of uncharged Ala or negatively charged Glu for positively charged Lys result in an inhibition of the protein translocation in vivo and a decrease in the efficiency of prePhoA insertion into liposomes in vitro. A similar effect was observed for prePhoA translocation in vivo, when the APL content was decreased by genetic manipulations, or for association of prePhoA with liposomes with the lower APL content in vitro. Moreover, the latter experiments showed that the dependence of prePhoA insertion on the APL content is determined by the signal peptide N-terminus charge. The in vitro results support a potential requirement of anionic phospholipids for prePhoA membrane insertion, independent of their requirement for the optimal function of Sec proteins, and also the possibility of a direct signal peptide N-terminus interaction with anionic phospholipids.
Molecular modeling and stereochemical analysis of the interaction of the signal peptide N-terminus with anionic phospholipids [56] by the example of prePhoA showed that hydrogen bonds are sufficient for the formation of a “signal peptide-phospholipid” complex, which needs less energy to insert into the lipid bilayer interior than is needed for insertion of its components separately. Lys(-20) in the signal peptide is able to form an additional ion pair with a negatively charged phosphate group of anionic phospholipid molecule [12]. This suggests that the positive charge of the signal peptide N-terminus enhances the efficiency of protein translocation due to stabilization of the “signal peptide-anionic phospholipid” complex during initiation of the translocation. The replacement of Lys(-20) by Ala or Glu prevents necessary electrostatic interaction and makes the above complex less stable. The resulting shortening of the lifetime of the “membrane-precursor” complex would also affect the kinetic parameters of protein translocation, decreasing its efficiency.
As a whole, the results in vivo are consistent with the proposed hypothesis [4]. They demonstrate the importance of interactions of the prePhoA signal peptide and anionic phospholipids at the initial step of secretion. However, it might be expected that mutant proteins with uncharged Ala or negatively charged Glu instead of positively charged Lys(-20)would preferably be translocated across the membrane having a low negative charge due to the lower PG and CL contents. However, in fact we have not found in vivo any expected differences between the efficiency of secretion of the wild type and mutant phosphatases depending on the APL content, at least in the range of their variation from 7 to 27%. This points either to the absence in vivo of the direct electrostatic interaction between the signal peptide and anionic phospholipids at the initial step of protein translocation or to the difficulty of its finding under the above APL variations. At present, this question is examined using the E. coli strain AD93, lacking phosphatidylethanolamine and subsequently containing 100% of APL in the membranes. It is quite possible that the interaction between the signal peptide and phospholipids in vivo could be determined by the lipid-dependent protein components of the secretory machinery, e.g., SecA protein. Now this problem is under study.
This work was supported by the Russian Foundation for Basic Research (grants 99-04-48153, 02-04-49765, and 02-04-06304) and the U. S. Civilian Research and Development Foundation for Independent States of the FSU (grant RB1-2038).
The authors are grateful to W. Dowhan for kindly providing the strains E. coli AD93 and E. coli HDL11 and to J. Beckwith for providing plasmid pBAD18.
REFERENCES
1.Pugsley, A. P. (1993) Microbiol. Rev.,
57,50-108.
2.Fekkes, P., and Driessen, A. J. M. (1999)
Microbiol. Mol. Biol. Rev., 63, 161-173.
3.Von Heijne, G. (1990) J. Membr. Biol.,
115, 195-201.
4.Nesmeyanova, M. A. (1982) FEBS Lett.,
142, 189-193.
5.Nesmeyanova, M. A., and Bogdanov, M. V. (1989)
FEBS Lett., 257, 203-207.
6.Inouye, S., Soberon, X., Franceschini, T. K. N.,
Itakura, K., and Inouye, M. (1982) Proc. Natl. Acad. Sci. USA,
79, 3438-3441.
7.Iino, T., Takahashi, M., and Sako, T. (1987) J.
Biol. Chem., 262, 7412-7417.
8.Bosch, D., Boer, P., Bitter, W., and Tommassen, J.
(1989) Biochim. Biophys. Acta, 979, 69-76.
9.Sasaki, S., Matsuyama, S.-I., and Mizushima, S.
(1990) J. Biol. Chem., 265, 4358-4363.
10.Phoenix, D. A., Kusters, R., Hikita, C.,
Mizushima, S., and de Kruijff, B. (1993) J. Biol. Chem.,
268, 17069-17073.
11.Mori, H., Araki, M., Hikita, C., Tagaya, M., and
Mizushima, S. (1997) Biochim. Biophys. Acta, 1326,
23-36.
12.Nesmeyanova, M. A., Karamyshev, A. L.,
Karamysheva, Z. N., Kalinin, A. E., Ksenzenko, V. N., and Kajava, A. V.
(1997) FEBS Lett., 403, 203-207.
13.De Vrije, G. J., Batenburg, A. M., Killian, J.
A., and de Kruijff, B. (1990) Mol. Microbiol., 4,
143-150.
14.Lill, R., Dowhan, W., and Wickner, W. (1990)
Cell, 60, 271-280.
15.Kusters, R., Dowhan, W., and de Kruijff, B.
(1991) J. Biol. Chem., 266, 8659-8662.
16.Kusters, R., Breukink, E., Gallusser, A., Kuhn,
A., and de Kruijff, B. (1994) J. Biol. Chem., 269,
1560-1563.
17.Batenburg, A. M., Demel, R. A., Verkleij, A. J.,
and de Kruijff, B. (1988) Biochemistry, 27,
5678-5685.
18.Cornell, D. G., Dluhy, R. A., Briggs, M. S.,
McKnight, C. J., and Gierasch, L. M. (1989) Biochemistry,
28, 2789-2797.
19.Demel, R. A., Goormaghtigh, E., and de Kruijff,
B. (1990) Biochim. Biophys. Acta, 1027, 155-162.
20.Mikhaleva, N. I., Kalinin, A. E., Molotkovsky,
Yu. G., and Nesmeyanova, M. A. (1997) Biochemistry (Moscow),
62, 184-190.
21.Kalinin, A. E., Karamyshev, A. L., and
Nesmeyanova, M. A. (1996) Biochemistry (Moscow), 61,
73-80.
22.Kalinin, A. E., Mikhaleva, N. I., Karamishev, A.
L., Karamisheva, Z. N., and Nesmeyanova, M. A. (1999) Biochemistry
(Moscow), 64, 1021-1029.
23.Hendrick, J. P., and Wickner, W. (1991) J.
Biol. Chem., 266, 24596-24600.
24.Kontinen, V. P., and Tokuda, H. (1995) FEBS
Lett., 364, 157-160.
25.Van Klompenburg, W., and de Kruijff, B. (1998)
J. Membr. Biol., 162, 1-7.
26.Shinkai, A., Hong Mei, L., Tokuda, H., and
Mizushima, S. (1991) J. Biol. Chem., 266, 5827-5833.
27.Cabelli, R. J., Dolan, K. M., Qian, L., and
Oliver, D. (1991) J. Biol. Chem., 266, 24420-24427.
28.Lill, R., Cunninghan, K., Brundage, L. A., Ito,
K., Oliver, D., and Wickner, W. (1989) EMBO J., 8,
961-966.
29.Bachmann, B. J. (1987) in Escherichia coli and
Salmonella typhimurium: Cellular and Molecular Biology
(Neidhardt, F. C., ed.) American Society for Microbiology, Washington,
pp. 1190-1219.
30.De Chavigny, A., Heacock, P. N., and Dowhan, W.
(1991) J. Biol. Chem., 266, 5323-5332.
31.Karamyshev, A. L., Shlyapnikov, M. G.,
Khmel'nitskii, M. I., Nesmeyanova, M. A., and Ksenzenko, V. N. (1994)
Mol. Biol. (Moscow), 28, 253-258.
32.Karamyshev, A. L., Kalinin, A. E., Khmel'nitskii,
M. I., Shlyapnikov, M. G., Ksenzenko, V. N., and Nesmeyanova, M. A.
(1994) Mol. Biol. (Moscow), 28, 253-258.
33.Guzman, L. M., Belin, D., Carson, M. J., and
Beckwith, J. (1995) J. Bacteriol., 177, 4121-4130.
34.Zaitsev, E. N., Zaitseva, E. M., Bakhlanova, I.
V., Gorelov, V. N., Kuzmin, N. P., Krykov, V. M., and Lantsov, V. A.
(1986) Genetics (Moscow), 22, 2721-2727.
35.Miller, J. H. (1972) Experiments in Molecular
Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor,
N. Y.
36.Torriani, A. (1966) in Procedures in Nucleic
Acid Research (Cantoni, G. L., and Davis, R., eds.) Harper and Row
Publishers, N. Y., pp. 224-234.
37.Sambrook, J., Fritsch, E. F., and Maniatis, T.
(1989) in Molecular Cloning: a Laboratory Manual, Cold Spring
Harbor Laboratory, N. Y., p. 1626.
38.Sanger, F., Nicklen, S., and Coulson, A. R.
(1977) Proc. Natl. Acad. Sci. USA, 74, 5463-5467.
39.Michaelis, S., Inouye, H., Oliver, D., and
Beckwith, J. (1983) J. Bacteriol., 154, 366-374.
40.Ames, G., Spudish, E., and Nicaido, H. (1968)
J. Bacteriol., 95, 833-843.
41.Fine, J. B., and Sprecher, H. (1982) J. Lipid
Res., 23, 660-663.
42.Chalvardjian, A., and Rudnicki, E. (1970)
Analyt. Biochem., 36, 225-226.
43.Killian, J. A., Keller, R. C. A., Struyve, M., de
Kroon, A., Tommassen, J., and de Kruijff, B. (1990)
Biochemistry, 29, 8131-8137.
44.Laemmli, U. K. (1970) Nature, 227,
680-685.
45.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) J. Biol. Chem., 193, 265-275.
46.Kamitani, S., Akiyama, Y., and Ito, K. (1992)
EMBO J., 11, 57-62.
47.Boyd, D., Guan, D.-D., Willard, S., Wright, W.,
Strauch, K., and Beckwith, J. (1987) in Phosphate Metabolism and
Cellular Regulation in Microorganisms (Torriani-Gorini, A., eds.)
American Society for Microbiology, Washington, D. C., pp. 89-93.
48.Michaelis, S., Hunt, J. F., and Beckwith, J.
(1986) J. Bacteriol., 167, 160-167.
49.Tanji, Y. J. G., Pollitt, S., and Inouye, M.
(1991) J. Bacteriol., 173, 1997-2005.
50.Vlasuk, G. P., Inouye, S., Ito, H., Itakura, K.,
and Inouye, M. (1983) J. Biol. Chem., 258, 7141-7148.
51.Hoyt, D. W., and Gierasch, L. M. (1991)
Biochemistry, 30, 10155-10163.
52.Kito, M., Aibara, S., Kato, M., and Hata, T.
(1972) Biochim. Biophys. Acta, 260, 475-478.
53.Yevdokimova, O. A., Nesmeyanova, M. A., and
Kulaev, I. S. (1978) Biokhimiya, 43, 1680-1687.
54.Zemlyanukhina, O. A., Kolycheva, V. V., and
Nesmeyanova, M. A. (1981) Biokhimiya, 46, 92-99.
55.Karamyshev, A. L., Karamysheva, Z. N., Kajava, A.
V., Ksenzenko, V. N., and Nesmeyanova, M. A. (1998) J. Mol.
Biol., 277, 859-870.
56.Kajava, A. V., Bogdanov, M. V., and Nesmeyanova,
M. A. (1991) J. Biomol. Struct. Dyn., 9, 143-147.