REVIEW: L-Lysine alpha-Oxidase: Physicochemical and Biological Properties
E. V. Lukasheva* and T. T. Berezov
Department of Biochemistry, Russian Peoples' Friendship University, ul. Miklukho-Maklaya 8, Moscow, 117198 Russia; fax: (095) 434-0412; E-mail: lukashev@aha.ru* To whom correspondence should be addressed.
Received April 17, 2002; Revision received June 10, 2002
This review summarizes data on the properties of L-lysine alpha-oxidase, an enzyme that belongs to the group of oxidases of L-amino acids. This enzyme acts virtually only on L-lysine with a rather low Km yielding alpha-keto-epsilon-aminocaproic acid. The decrease in the level of the essential amino acid L-lysine and the formation of hydrogen peroxide during the reaction possibly provide the basis for the unique properties of L-lysine alpha-oxidase: cytotoxic, antitumor, antimetastatic, antiinvasive, antibacterial, and antiviral activities, as well as an immunomodulating effect. Native L-lysine alpha-oxidase and its immobilized forms are promising tools for determination of concentration of L-lysine in various biological materials.
KEY WORDS: L-lysine alpha-oxidase, L-amino acid oxidase, immobilized enzymes, L-lysine assay, enzymes in medicine, antitumor enzyme, antiviral enzyme, antibacterial enzyme, antiinvasive effect, antimetastatic effect, soluble enzyme conjugates, enzyme conjugated with monoclonal antibodies to tumor cell receptors
Abbreviations: LO) L-lysine alpha-oxidase.
Since the middle of the last century some enzymes have been of special
interest for investigators due to the possibility of their application
in medicine, particularly in oncology. The requirements for enzymes of
medical purposes formulated in [1, 2] were taken as the basis for the selection of
antitumor enzymes. Some lines of tumor cells are selectively sensitive
to the decrease in the level of certain metabolites. Essential amino
acids, including L-lysine, cannot be synthesized in the body [3]. Thus, enzymes capable of cleaving these amino
acids are promising for investigation aimed at their application in
oncology. Several groups of enzymes catalyzing irreversible cleaving of
L-amino acids have been described in the literature. One of them
includes oxidases of L-amino acids that have been known since the 1930s
as enzymes catalyzing oxidative deamination of the alpha-amino
group [4].
L-Lysine alpha-oxidase (LO) is one of the representatives of this group. This enzyme was first isolated in the laboratory of Prof. K. Soda (Japan) from Trichoderma viride Y 244-2 [5, 6]. Later, a domestic producer of this enzyme, Trichoderma harzianum Rifai, was found by researchers of Peoples' Friendship University (School of Medicine, Department of Biochemistry) [7]. During the enzymatic reaction, oxygen is consumed yielding ammonia, hydrogen peroxide, and alpha-keto-epsilon-aminocaproic acid that can be transformed spontaneously into a cyclic derivative, Delta´-piperidine-2-carboxylate:
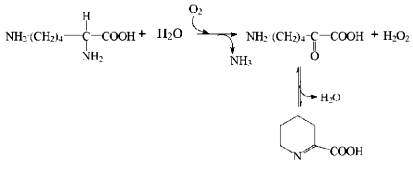
Purification of L-lysine alpha-oxidase. Japanese researchers isolated the homogeneous enzyme with 8% yield and the specific activity of 66 U/mg from the extract of the wheat bran fungal culture of Trichoderma viride Y 244-2 using an eight-step method for purification [6]. In our laboratory, LO from Trichoderma harzianum Rifai was originally isolated from the extract of a surface-cultivated fungal culture using a four-step method with 22.4% yield and the specific activity of 29 U/mg [8]. Since wide preclinical investigations required a large amount of the homogeneous enzyme, a method of submerged cultivation of the fungus Trichoderma harzianum Rifai and a four-step method of the enzyme purification were developed in our chair in collaboration with NPO Ferment (Vilnius, Republic of Lithuania). The method of purification allowed isolation of the enzyme with 56% yield and the specific activity of 31.5 U/mg using the affinity chromatography [9].
Investigation of physicochemical properties. The molecular weight of the enzyme determined by two independent methods, gel filtration on Sephadex G-200 and PAGE, was shown to be 120 kD, the protein molecule consisting of two identical subunits of 60 kD [10]. The corresponding values for LO from Trichoderma viride Y 244-2 were about 116 and 56 kD [6]. The isoelectric points of LO from the two sources were virtually the same, 4.25. Later, a second form of LO was isolated from the submerged-cultivated fungus Trichoderma harzianum Rifai that is likely to be a product of proteolysis of the native LO; the molecular weight of the enzyme and pI value being 100 kD and 5.6, respectively [11]. The enzymes from both sources contained one molecule of FAD per subunit as the coenzyme. Their optical absorption spectra corresponded to the spectra of FAD-dependent enzymes and exhibited maxima at 360 and 460 nm, as well as a peak at 280 nm that is characteristic for all proteins [6, 10]. The LO molecule can contain from 3 to 98 carbohydrate residues depending on the source of the enzyme and method of its purification [12]. Determination of the number of free amino groups on the surface of the molecule of LO from Trichoderma harzianum Rifai using 2,4,6-trinitrobenzenesulfonic acidshowed that only 3 of 43 lysine residues revealed in the LO molecule by amino acid analysis [13] were located on the surface of the enzyme molecule. Presumably, some of the amino groups are inaccessible to the reaction due to intramolecular interactions. The low accessibility of the amino groups of LO is easy to explain. It is known from the literature that alpha-keto acids formed in the reactions of oxidative deamination are capable of reacting with the amino groups of proteins [13]. This fact suggests that the spatial structure of oxidases of L-amino acids tended to minimization of the surface amino groups during evolution. If the LO molecule bound to the product, alpha-keto-epsilon-aminocaproate, during the reaction, the modified protein molecule would be ready for modification again, because the number of amino groups in the molecule would be maintained constant. This would lead to an unlimited increase in the molecular weight of LO during the reaction. Thus, we can conclude that the small number of the accessible amino groups is an important characteristic of this protein connected with the nature of the reaction product [12]. Investigation of the enzymes from two sources showed that they have different structure. The molecule of LO from Trichoderma harzianum Rifai contains 12% of alpha-helix and 33% of beta-form, the contribution of the corresponding structures in the molecule of LO from Trichoderma viride Y 244-2 constituting 13 and 18%, respectively [14]. Amino acid analysis revealed a significant difference in the content of some amino acids. The enzyme from Trichoderma harzianum Rifai contained about 2.6-fold as many glycine residues, the number of arginine and tryptophan residues being about 4- and 2-fold less than that in the enzyme from Trichoderma viride Y 244-2 [14].
Investigation of the pH dependence of the activity of LO from Trichoderma harzianum Rifai using the polarographic method revealed a wide plateau in the range of pH from 4.5 to 10.5 [15].
Investigation of the substrate specificity of LO from Trichoderma harzianum Rifai showed that the enzyme catalyzes mainly oxidative deamination of L-lysine (Table 1) [16, 17]. Amino acids bearing positive charge on the end of the chain of 4-6 carbon atoms, such as ornithine, arginine, and S-aminoethyl-cysteine are subjected to the reaction with much lower rates, and the observed Km values (Table 1) indicate that the affinity of the enzyme to L-lysine is significantly higher than to other substrates.
Table 1. Substrate specificity of L-lysine
alpha-oxidase from Trichoderma harzianum Rifai* [16]
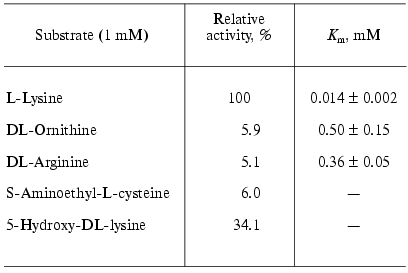
*L-Lysine alpha-oxidase did not exhibit activity in the
presence of 1 mM of the following substances: DL-phenylalanine,
DL-alanine, DL-leucine, DL-histidine, DL-asparagine, L-glutamine,
L-citrulline, D-arginine, D-lysine, D-ornithine, L-lysinamide,
L-albizziin
H2N-C(O)-NH-CH2-CH(NH2)-COOH,
N-alpha-acetyl-L-lysine, N,N´-dicarbobenzoxy-DL-ornithine,
L-canavanine
NH=C(NH2)-NH-O-(CH2)2-CH(NH2)-COOH.
L-Lysine alpha-oxidase from Trichoderma viride Y244-2 (Japan) has a wider substrate spectrum and a lower Km value to L-lysine [6]. In terms of the substrate specificity, LO from Trichoderma harzianum Rifai is similar to L-lysine-monooxygenase that acts on lysine yielding other reaction products. Like other L-amino acid oxidases, LO from both sources exhibit a high stereospecificity, being absolutely inert towards D-isomers of the amino acids that are employed as both substrates and inhibitors.
Effectors of the enzymatic reaction. Among ions of bivalent metals, Hg2+, Zn2+, and Cd2+ exhibited the most pronounced inhibiting effect, while Mn2+ and Co2+ increased the rate of L-lysine oxidation. Introduction of additional amounts of FAD did not increase the activity, this correlating with the fact that the protein part of the enzyme is completely saturated with the tightly bound coenzyme.
Products of enzymatic reactions often exhibit inhibitory properties. One of the product of the reaction investigated, hydrogen peroxide, causes 15% decrease in the rate of the reaction. To prevent the inhibitory effect of H2O2, catalase was added into the reaction mixture. This resulted in 1.5-fold increase in the initial rate of the enzymatic reaction due to the supply of oxygen released during the decomposition of H2O2 caused by catalase. We assumed that the rate of the reaction could be also increased by the introduction of the electron carrier facilitating the interaction between the enzyme and oxygen. However, no changes in the reaction rate were observed in the presence of phenazine methosulfate (PMS) that was used as the carrier. Oxidants, such as potassium ferricyanide, did not effect the enzymatic reaction, this suggesting that the oxidant could not interact directly with the enzyme. Simultaneous addition of the oxidant K3[Fe(CN)6] and the electron carrier PMS resulted in 2-fold increase in the reaction rate. Analysis of the data on the structure of the substrate analogs and the reaction products affecting the rate of the enzymatic reaction suggests that the active site of LO contains both positively and negatively charged groups capable of binding the carboxyl group of the substrate as well as the 5-7 Å removed terminal amino group [16].
Effect of modification of some amino acid residues on the enzymatic activity of L-lysine alpha-oxidase. Considering that enzymes having a number of characteristics similar to LO (D-amino acid oxidase from goat kidneys and L-amino acid oxidase from snake venom) contain arginine residue in their active sites, we decided to investigate the role of the arginine residue in the catalytic activity of LO. 2,3-Butanedione was used as the reagent providing selective modification of arginine residues under rather mild conditions. Decrease in the activity of L-lysine alpha-oxidase was observed during 10-20 min, and then the activity remained virtually constant. Amino acid analysis of the modified enzyme showed that 4 h after the initiation of the reaction the number of the arginine residues modified with 2,3-butanedione constituted 37% of the total number of arginine residues in the enzyme molecule. The irreversible inactivation of LO constituted 18% of the activity and the reversible part was about 50%. Modification of the enzymes containing the arginine residue in their active sites with 2,3-butanedione results in the irreversible loss of about 90% of the activity during 20-30 min. Thus, our results do not allow making the conclusion that arginine plays an essential role in the active site of LO for the catalytic activity of the enzyme [18]. Later, it was shown that the amino and carboxyl groups were not essential for the catalytic activity of the enzyme [12].
Investigation of enzyme stability. LO from Trichoderma harzianum Rifai exhibits a relatively high stability: after 3 h of incubation at 60°C the enzyme retains more than 60% of its activity. The activity decreases only during the first 25 min of the incubation, and then no significant changes in activity is observed for the next 3 h. At 65 and 70°C fast inactivation in the beginning of the incubation with the subsequent slow inactivation are observed. At 75 and 80°C the inactivation of LO from Trichoderma harzianum Rifai proceeds very quickly. Comparing with LO from Trichoderma viride Y 244-2 shows that this enzyme has a lower thermal stability. Thermal stability of LO significantly increases in diluted solutions.
The character of the kinetic curves of inactivation of LO [19, 20] suggests that the dissociation of the enzyme into subunits is the stabilizing factor decelerating significantly its thermal inactivation. Elucidation of the mechanism of the inactivation of LO is of practical interest, indicating that the enzyme should be stored as the diluted solution. Being stored lyophilized at 4°C or as a frozen solution at -18°C, the enzyme retained its activity virtually constant for a year. Incubation of LO at 37°C and pH 7.4 or 6.6 for 24 h did not result in a decrease in the enzymatic activity. The enzyme retained 60% of its activity after incubation in an alkaline medium at pH 9.5 (borate buffer), this characterizing the enzyme positively in terms of its further applicability.
Application of L-lysine alpha-oxidase for assaying of L-lysine. Being an essential amino acid, L-lysine is an important component of nutrient additions, mixtures for parenteral feeding, nutrient media for cell and tissue cultures, etc. Its wide industrial application requires methods for easy and specific determination of L-lysine concentration in culture liquids and final products. Since L-lysine alpha-oxidase acts selectively on L-lysine, virtually not affecting the rate of oxidative deamination of other natural amino acids, structural analogs of L-lysine, different analytical methods employing this enzyme were created for assaying of L-lysine [6, 21, 22]. Immobilized enzymes have some advantages compared to soluble ones in the case of their application in analytical purposes. Therefore, methods of simultaneous immobilization of LO and horseradish peroxidase on porous membrane supports were elaborated [23]. The membranes can be used repeatedly; their sensitivity depending on the system varies in the range of 20-120 µM, and the reaction time is 2-20 min [23]. Application of enzyme immobilized on gelatin using a flow-type system with a platinum electrode allows determination of L-lysine in the range of 0.2-4.0 mM [24]. Application of LO immobilized on ammonia-sensitive electrodes for assaying of L-lysine has a significant limitation due to the capability of reacting with cations of some metals [25]. Production of hydrogen peroxide during the L-lysine alpha-oxidase reaction allows amperometric determination of this substance [26]. A passive adsorption of LO on a platinum electrode covered with 1,2-diaminobenzene by the electropolymerization method yielded good results: the linear region for determination of L-lysine was 10-1000 µM, the lowest detectable concentration of L-lysine being 0.2 µM [27].
STUDY OF THE BIOLOGICAL EFFECT OF L-LYSINE alpha-OXIDASE
in vitro
Using radioactive isotopes, LO was shown to suppress DNA, RNA, and protein synthesis in leukemia L-5178Y [28], human ovary carcinoma (CaOv), and Burkitt lymphoma [29, 30] tumor cells in vitro.
Flow-type cytofluorimetric studies of the detailed cell mechanisms of the effect of LO on the stages of the proliferative cycle of cultivated Burkitt lymphoma cells showed that the enzyme at the concentration of 10-3 U/ml after 48 h of incubation blocked the transition of the cells from S phase to G2/M growth phase. The number of cells in G2/M phase decreased almost by 17% compared to that of the control, the number of cells in G0/G1 and S growth phases increasing by 6.3 and 10.2%, respectively [31]. These data indicate that LO affects S growth phase of Burkitt lymphoma cells.
Experiments in vitro on L5178Y cells showed that besides the cleaving of L-lysine, the cell growth was also decreased by H2O2 produced during the enzymatic reaction [6]. However, there are data indicating that LO suppresses effectively the cell growth (MM1 cell line) even in the presence of catalase [32, 33]. A positive characteristic of LO is its an antiinvasive effect demonstrated in vitro on MM1 cells in the presence of catalase, suggesting that the decrease in L-lysine concentration in the medium is the main reason for the antiinvasive effect in vitro [33].
Elaboration of a system for therapy of leukemia based on immobilized L-lysine alpha-oxidase. Since the experience of application of L-asparaginase, the only enzyme employed in the therapy of tumors, showed that the injection of this preparation often caused undesirable side effects including the anaphylactic shock. Reiken and Bredis suggested using LO co-immobilized with catalase as the extracorporal shunt, so as to avoid the ingress of the enzyme into the organism [34]. While working in the pulsating regime with recycling, the method resulted in the removal of about 40% of L-lysine from the solution simulating blood plasma for 4 h. However, there have been no reports in literature on the practical application in oncology of this extracorporal system with two immobilized enzymes.
Antibacterial effect of L-lysine alpha-oxidase on the rec- mutant of Bacillus subtilis was demonstrated in [35]. The antibacterial activity of LO decreased in vitro on the addition of catalase, the enzyme catalyzing decomposition of hydrogen peroxide. Thus, the decrease in the antiproliferative and antibacterial activities in the presence of catalase suggests that the destruction of the DNA molecule by hydrogen peroxide is likely to be the primary effect of the enzyme on the cell.
Later, research of our chair demonstrated that LO exhibited antiviral activity. It inhibited the reproduction of type I Herpes simplex virus. In the presence of 10-2 U/ml (0.3 µg/ml) of the enzyme, the cytopathic effect of type I Herpes simplex virus on the cells cultivated in vitro decreased almost 105-fold. Besides, LO effectively suppresses the expression of the viral antigens. Therefore, it was concluded that highly purified preparations of LO are more effective agents suppressing the reproduction of Herpes simplex virus compared to luteolin and acyclovir [36, 37].
Together with the works on investigation of the effect of LO on Herpes simplex virus, the influence of LO on other viruses was studied. For example, it was shown that the presence of 10-4-10-5 U/ml of LO in the culture liquid completely suppressed human immunodeficiency virus [38]. Increase in concentration of LO in the experiments resulted in the cytotoxic effect on the cells.
STUDY OF BIOLOGICAL EFFECT OF L-LYSINE alpha-OXIDASE in
vivo
Time-dependence of L-lysine alpha-oxidase concentration in plasma. The maximal activity of LO in blood plasma of experimental mice was observed 2 h after an intravenous injection of high doses of LO (300 U/kg) [28]. Ten hours after the injection about 10% of the maximal activity remained in the plasma. However, based on the total amount of the injected enzyme, the half-life time of LO was 2 h, less than that compared of other antitumor enzymes studied in experiments on animals.
Change in the level of L-lysine in plasma. A single intravenous injection of 70 U/kg of LO to BDF1 miceresulted in a decrease in the level of L-lysine in the plasma from 0.33 mM to a very small level that remained constant for 12 h. Complete recovery of the original concentration of L-lysine was observed only after 24 h [28]. If the same dose were injected intraperitoneally, the level of L-lysine decreased to 15% of the control level after 1 h, reaching undetectably small values after only 6 h. The level of this amino acid recovered to 50% of the original value after 24 h. The data on monitoring of the dynamic of the changes in concentration of L-lysine in blood plasma indicate that LO exhibits high activity in vivo.
Immunomodulating action of L-lysine alpha-oxidase was demonstrated using the model of the endogenous colony formation of the mouse spleen surface after the injection of LO solution according to a specific plan with subsequent sub-lethal radiation treatment of the animals [39].
Antiviral therapeutic effect of LO on the development of the genital herpetic infection of guinea pigs was demonstrated in [40].
Antitumor effect of L-lysine alpha-oxidase from Trichoderma viride Y 244-2 was investigated on mice bearing grafted L1210 tumor. It was shown that daily single injections of 70 U/kg of LO for 5 days starting with the 1st day of grafting of the tumor increased the lifetime of the mice by 34-48% compared to that of the untreated animals [28].
As known from the literature, LO exhibits a rather high cytotoxic effect on a number of lines of tumor cells, but, in contrast to L-asparaginase, LO exhibits the antileukemiaand antimetastatic effects in vivo in rather small effective doses [28, 32]. These properties of LO, as well as the fact that the spectrum of its antitumor effect of this enzyme could differ from that of L-asparaginase gave a rise to further investigations of the antitumor effect of LO from Trichoderma harzianum Rifai in vivo.
The activity of LO towards hemoblastoses and ascites tumors of mice and rats is presented in Tables 2 and 3 [41]. The ratio T/C (%) (treatment/control) was calculated as the ratio of the mean lifetime of the experimental animals to that of the control animals multiplied by 100%. The mean lifetime was determined after death of all mice in the group. Inhibition of the tumor growth (TGI) was calculated as follows: TGI(%) = [(Vexp - Vc)/Vc]*100%, where Vexp and Vc are the mean volumes of the tumors in the experiment and in the control, respectively. The mean volume of the tumors was defined as Vmean = a*b*c (cm3). Effects were considered to be significant at TGI > 70% and T/C = 136-150%.
Table 2. Antitumor effect of L-lysine
alpha-oxidase from Trichoderma harzianum Rifai on strains
of hemoblastoses and ascites tumors compared to that of L-asparaginase
from E. coli [41]
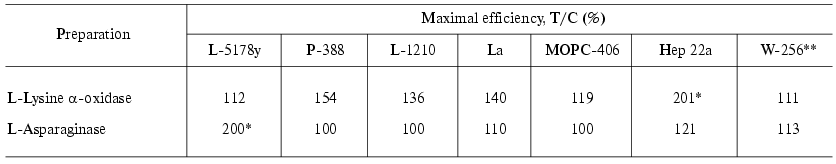
*Complete remission 29-66%.
**Rat tumor (in other cases mouse tumors were studied).
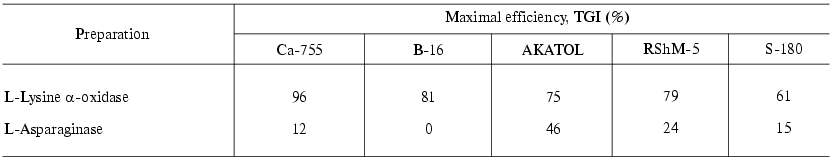
Table 3. Antitumor effect of L-lysine
alpha-oxidase from Trichoderma harzianum Rifai on strains
of solid tumors compared to that of L-asparaginase from E. coli
[41]
It is seen that the effect of LO in the case of hemoblastoses of mice is low. LymphoadenosisL-5178Y, being highly sensitive to L-asparaginases, was not affected by LO. In the cases P-388, L-1210, and La the effect of LO on the T/C value did not exceed 136-154%. In the case of plasmocytoma MOPS-406 an effect of LO was virtually absent. Walker carcinosarcoma W-256 was also unsusceptible to LO. The best results were obtained on ascites hepatoma 22. Application of LO resulted in a significant increase in the lifetime of the mice (T/C = 201%); 29-66% of the mice completely recovered from the tumor. The mice were considered to be recovered (CR, complete remission) if they were alive 120 days after the inoculation of the tumor.
L-Asparaginase was highly effective in curing of lymphoadenosis L-5178Y, the T/C value and recovery percentage reaching 200 and 70%, respectively. In the cases of other hemoblastoses and ascites tumors, the effect was small or absent.
Among solid tumors, a pronounced antitumor effect of LO was observed in the case of Ca-755, the TGI value reaching 90-96%. In the cases of B-16, RShM-5 and AKATOL the effect did not exceed 75-81%. A slight effect was observed on S-180 (TGI = 61%). L-Asparaginase had virtually no affect the growth of the mouse solid tumors investigated.
Therefore, considering the defined parameters of the efficiency, LO-sensitive tumors constitute five of 12 tested tumors: ascites hepatoma 22A, mammary gland adenocarcinoma Ca-755, melanoma B-16, uterine cervix cancer RSHM-5, large intestine adenocarcinoma AKATOL.
Antimetastatic effect of L-lysine alpha-oxidase was demonstrated on mice bearingLewis lung carcinoma [32]. On the injection of LO the quantity and volume of metastasesdecreased significantly in the unoperatedanimals as well as in the case of the removal of the tumor, the antimetastatic effect increasing with the increase in the doses of the injected LO. Study of the activity of the enzymes involved in the adenosine exchange in the alveolar macrophages that contact directly the metastatic cells in the lungs of the mice showed that the injection of LO increased the activity of adenosine deaminase and decreased the activity of 5´-nucleotidase compared to the corresponding values of the untreated animals. This created conditions for the decrease in the intracellular concentration of adenosine, an inhibitor of the functional activity of macrophages. However, the decrease in the activity of 5´-nucleotidase located on the surface of macrophages suggests that LO could influence the cell membrane of the macrophages.
PREPARATION OF CONJUGATES OF LO WITH ANTIBODIES AND STUDY OF
THEIR PHYSICOCHEMICAL AND BIOLOGICAL PROPERTIES
Creation of modern medicines requires, first, selection of highly selective preparations, and second, realization of the directed transport of the drug to the affected tissues. Our studies demonstrated that native LO acts selectively on its substrate, so its action on any other substances in an organism aside from free L-lysine is excluded. As for the possibility of the directed transport in an organism, the free enzyme, naturally, is not endowed with such a property. A common way to endow a drug with the affinity to a definite organ is its binding to a certain vector molecule. Antibodies exhibit the highest binding constants to the ligands of target cells, so they were chosen for preparation of conjugates with the enzymes. Approaching the creation of antitumor drugs of the directed action based on enzymes and antibodies, we formulated the following principles to the methods of conjugation: the conjugation must result in the minimal losses of the enzymatic activity of the included enzyme (1), of the immune activity of the antibodies (2), and of the cytotoxic activity of the components of the conjugate (3).
The work on creation of the conjugates of LO with monoclonal antibodies included two steps. In the first step, the methods of conjugation of LO were developed using nonspecific antibodies, and the most promising of them were selected [42]. In the second step, using glutaraldehyde as the cross-linking agent, LO was bound to the ICO-80 monoclonal antibodies to the CD-5 receptor of the Yurkat cell line with 65% yield in the enzymatic activity and a virtually complete retention of the immune activity [43].
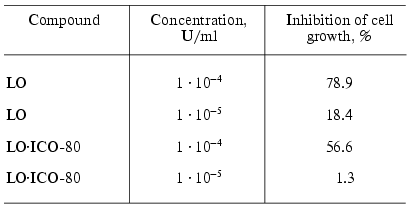
Investigation of the cytotoxic effect of the conjugate of LO and the monoclonal antibodies on Yurkat line cells showed that the double conjugates LO*ICO-80 possessed cytotoxic activity that was slightly decreased compared to the original enzyme (Table 4). It should be noted that the cytotoxic activity of the conjugates with the antibodies was studied on the tissue culture, but in vivo the efficiency of the conjugates with the specific antibodies can increase due to the affinity to certain types of cells. Thus, we can assume that further studies of conjugates of enzymes with antibodies, especially the conjugates of LO with the specific antibodies, can yield promising results on the way to creation of the active antitumor preparation.
Table 4. Inhibition of growth of Yurkat line
cells in the presence of different concentration of the conjugate of
L-lysine alpha-oxidase with the monoclonal antibodies [43]
The data presented in this review indicate that LO satisfies the demands for enzyme preparations for use in oncology [1, 2], exhibiting high stability, strong binding with the coenzyme, and high selectivity of action. Positive effects of both the native enzyme and its immobilized forms observed in our experiments give a hope that the scientifically based industrial production of standard enzyme solutions will provide doctors with a strong weapon against cancer diseases.
REFERENCES
1.Berezov, T. T. (1971) Vestnik AMN SSSR, No.
11, 35-46.
2.Holsenberg, J. S. (1982) Ann. Rev. Biochem.,
51, 795-809.
3.White, A., Handler, P., Smith, E., Hill, R., and
Lehman, I. (1981) in Principles of Biochemistry [Russian
translation], Vol. 2, Mir, Moscow, pp. 955-958.
4.Meister, A., and Welner, D. (1963) in The
Enzymes (Boyer, P. D., ed.) Academic Press, New York, pp.
609-648.
5.Kusakabe, H., Kodama, K., Machida, H., and
Kuninaka, A. (1979) Agr. Biol. Chem., 43, 337-343.
6.Kusakabe, H., Kodama, K., Kuninaka, A., Yoshino,
H., and Soda, K. (1980) J. Biol. Chem., 255, 976-981.
7.Smirnova, I. P., and Khaduev, S. Kh. (1984)
Mikrobiologiya, 53, 163-164.
8.Khaduev, S. Kh., Lukasheva, E. V., Smirnova, I. P.,
and Berezov, T. T. (1985) Vopr. Med. Khim., 31,
130-134.
9.Laugalene, N. F., Vesa, V. S., Yankyavichene, R.
P., Puodzhyute, S. P., Sudzhyuvene, O. F., Peslyakos, I. I., and
Khaduev, S. Kh. (1990) Vopr. Med. Khim., 36, 88-90.
10.Khaduev, S. Kh., Smirnova, I. P., Lukasheva, E.
V., et al. (1988) Vopr. Med. Khim., 34, 97-100.
11.Vesa, V. S., Lukasheva, E. V., Khaduev, S. Kh.,
and Berezov, T. T. (1996) Byull. Eksp. Biol. Med., 4,
404-406.
12.Lukasheva, E. V. (2002) Doctoral dissertation [in
Russian], Russian Peoples' Friendship University, Moscow.
13.Hafner, E. W., and Wellner, D. (1971) Proc.
Nat. Acad. Sci. USA, 68, 987-991.
14.Khaduev, S. Kh., Lukasheva, E. V., Vesa, V. S.,
Oikava, T., Esaki, N., Soda, K., Adachi, O., Yagi, K., and Berezov, T.
T. (1992) Vopr. Med. Khim., 38, 46-48.
15.Lukasheva, E. V., Vesa, V. S., Korpela, T. K.,
and Berezov, T. T. (1991) Vopr. Med. Khim., 37,
68-70.
16.Berezov, T. T., and Lukasheva, E. V. (1988)
Biochem. Int., 17, 529-534.
17.Lukasheva, E. V., and Berezov, T. T. (1988)
Prikl. Biokhim. Mikrobiol., 24, 459-465.
18.Lukasheva, E. V., Vesa, V. S., Korpela, T. K.,
and Berezov, T. T. (1992) Biokhimiya, 57, 452-455.
19.Lukasheva, E. V., Dzhukovskii, A. P., Soda, K.,
and Berezov, T. T. (1991) Biokhimiya, 56, 1069-1076.
20.Lukasheva, E. V., Dzhukovskii, A. P., and
Berezov, T. T. (1994) in Modern Enzymology: Problems and
Trends (Kurganov, B. I., Kochetkov, S. N., and Tishkov, V. I.,
eds.) Nova Science Publishers, New York, pp. 593-597.
21.Lukasheva, E. V., Berezov, T. T., and Smirnova,
I. P. (1987) Vopr. Med. Khim., 33, 127-132.
22.Kusakabe, H., Kodama, K., Kuninaka, A., Yoshino,
H., and Soda, K. (1979) Agr. Biol. Chem., 43,
1749-1752.
23.Lukasheva, E. V., Rubtsova, M. Yu., Kovba, G. V.,
Berezov, T. T., and Egorov, A. M. (1997) Vopr. Med. Khim.,
42, 566-575.
24.Romette, J. L., Yang, J. S., Kusakabe, H., and
Thomas, D. (1983) Biotechnol. Bioeng., 25, 2557-2566.
25.Saurina, J., Hernandez-Cassou, S., Alegret, S.,
and Fabregas, E. (1999) Biosens. Bioelectron., 14,
67-75.
26.Saurina, J., Hernandez-Cassou, S., Alegret, S.,
and Fabregas, E. (1999) Biosens. Bioelectron., 14,
211-220.
27.Kelly, S., Curulli, A., O'Sullivan, C.,
Guilbault, G. G., and Palleschi, G. (1998) Biosens.
Bioelectron., 13, 1245-1250.
28.Kusakabe, H., Kodama, K., Kuninaka, A., Yoshino,
H., and Soda, K. (1980) Agr. Biol. Chem., 44,
387-392.
29.Khaduev, S. Kh., Zhukova, O. S., Dobrynin, Ya.
V., Soda, K., and Berezov, T. T. (1986) Byull. Eksp. Biol. Med.,
5, 603-604.
30.Khaduev, S. Kh., Zhukova, O. S., Dobrynin, Ya.
V., Soda, K., and Berezov, T. T. (1987) Byull. Eksp. Biol. Med.,
4, 458-460.
31.Zhukova, O. S., Khaduev, S. Kh., Dobrynin, Ya.
V., Smirnova, I. P., Lukasheva, E. V., and Berezov, T. T. (1985)
Eksp. Onkol., 7, 42-44.
32.Khaduev, S. Kh., Umanskii, V. Yu., Zaletok, S.
P., Balitskii, K. P., Berdinskikh, N. K., and Berezov, T. T. (1990)
Byull. Eksp. Biol. Med., 5, 458-459.
33.Khaduev, S. Kh., Umanskii, V. Yu., Vesa, V. C.,
Sinkai, K., Akedo, Kh., and Berezov, T. T. (1991) Byull. Eksp. Biol.
Med., 10, 419-422.
34.Reiken, S. R., and Bredis, D. (1990)
Biotechnol. Bioeng., 35, 260-267.
35.Kusakabe, H., Sugi, M., Kodama, K., Kuninaka, A.,
Yoshino, H., and Soda, K. (1979) Agr. Biol. Chem.,
43, 1371-1373.
36.Berezov, T. T., Zverev, V. V., Zaitsev, I. Z.,
and Alekseev, S. B. (1997) Byull. Eksp. Biol. Med., 123,
36-38.
37.Smirnova, I. P., Diordittsa, S. V., Alekseev, S.
B., and Zaitsev, I. Z. (1998) Vopr. Med. Khim., 44,
384-387.
38.Berezov, T. T., Smirnova, I. P., Alekseev, S. B.,
Stepanova, L. G., Zverev, V. V., Gol'tsov, V. A., Andzhaparidze, S. G.,
and Vesa, V. S. (1994) RF Patent No. 2022011.
39.Alekseev, S. B., Smirnova, I. P., and Berezov, T.
T. (1997) Vopr. Med. Khim., 43, 112-115.
40.Smirnova, I. P., Alekseev, S. B., Diordittsa, S.
V., Vesa, V. S., and Zaitsev, I. Z. (1999) Byull. Eksp. Biol.
Med., 128, 654-656.
41.Treshalina, H. M., Lukasheva, E. V., Sedakova, L.
A., Firsova, G. A., Guerassimova, G. K., Gogichaeva, N. V., and Berezov
T. T. (2000) Appl. Biochem. Biotechnol., 84, 267-273.
42.Gogichaeva, N. V., Lukasheva, E. V., Gavrilova,
E. M., Smirnova, I. P., Egorov, A. M., and Berezov, T. T. (2000)
Vopr. Med. Khim., 46, 410-418.
43.Zhukova, O. S., Gogichaeva, N. V., Lukasheva, E.
V., and Berezov, T. T. (2001) Vopr. Med. Khim., 47,
588-592.