Soluble Tankyrase Located in Cytosol of Human Embryonic Kidney Cell Line 293
A. N. Kuimov1* and S. M. Terekhov2
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3181; E-mail: kuimov@genebee.msu.su2Medico-Genetic Scientific Center, Russian Academy of Medical Sciences, ul. Moskvorech'e 1, Moscow, 115478 Russia
* To whom correspondence should be addressed.
Received August 27, 2002; Revision received November 11, 2002
We studied the subcellular localization of tankyrase in primary and immortalized human cell cultures. In embryonic kidney cell line 293 the enzyme was excluded from the nuclei and distributed in fractions of soluble cytosolic proteins and low-density microsomes. Newly revealed cytosolic tankyrase in its poly(ADP-ribosyl)ated form was passed through a Sepharose 2B column and eluted as an apparently monomeric protein. The cytosolic localization of the enzyme correlated with its relatively high activity in the 293 cell line in comparison to eight other studied cell types.
KEY WORDS: tankyrase, poly(ADP-ribose), ADP-ribosylation
Abbreviations: GFP) green fluorescent protein; IRAP) insulin-responsive aminopeptidase; NuMA) nuclear/mitotic apparatus protein; PARP) poly(ADP-ribose)polymerase; SAM) sterile alpha motif; TAB182) 182-kD tankyrase-binding protein; TNKL) tankyrase-like protein or tankyrase-2; TRF1) telomeric repeat binding factor-1.
Tankyrase is a 142-kD polypeptide (in its non-modified form) discovered
in 1998 by yeast two-hybrid screen as an enzyme bound to TRF1, a
telomeric protein [1]. Along with domains of
protein-protein interactions, one of them containing 24 ankyrin
repeats, tankyrase has a PARP domain with homology to the catalytic
domain of NAD+-ADP-ribosyltransferase (EC 2.4.2.30). A
product of the intrinsic enzyme activity is a protein-bound
poly(ADP-ribose) chain, which is built of units originating from
cleaved NAD+. The initially identified protein substrates
were TRF1 and tankyrase itself. The poly(ADP-ribosyl)ation reduces
DNA-binding activity of TRF1, the consequences of the modification for
dissociated acceptor protein and its fate being unknown. In telomerase
expressing cells the release of TRF1 from telomeres induces an increase
in telomere length. Because of this feature tankyrase is thought to be
a positive regulator of telomerase. The mechanism of tankyrase
activation involved in its regulatory action is not still clear [2].
Later tankyrase was found also on nuclear membrane [3] and in Golgi and membrane GLUT4 vesicles, storage of insulin-dependent glucose transporter [4]. Another protein poly(ADP-ribose) acceptor is an insulin responsive amino peptidase, IRAP. In 2002 tankyrase was shown to bind a newly discovered protein component of cortical actin cytoskeleton, TAB182 [5], as well as a nuclear/mitotic apparatus protein NuMA during mitosis [6]. Tankyrase is now considered a signaling molecule targeted to several subcellular compartments [2].
Recently we simultaneously with other laboratories found another enzyme of similar structure and activity named TNKL (tankyrase-like protein) or tankyrase-2 [7, 8] (Swissprot Q9H2K2). Gene tnkl called also tnks2 or tank2 is mapped on chromosome 10, while tankyrase-1, a name designating the first discovered isoenzyme [2], is encoded by a gene on chromosome 8. Tankyrase-2 has also been observed in various membrane subcellular structures [4, 9] and may be complexed with the telomeric protein in vitro [2, 10, 11]. It poly(ADP-ribosyl)ates itself and TRF1. Overexpression of tankyrase-2 tagged with nuclear localization signal, as well as tankyrase-1 under the same conditions, releases TRF1 from telomeres and induces an increase in telomere length [2].
Tankyrase-1 has been shown to depend on stimulation by insulin and growth factors via the Ras-MAPK signaling pathway [4], although these data have not yet been confirmed in other laboratories. It is unknown whether the hormones also regulate tankyrase-2 [11]. On the other hand, tankyrase-2 interacts with an adaptor protein Grb14, which contains an Src homology domain 2 [9]. Indirectly this finding indicates that tankyrase-2 may depend on signals transduced by protein tyrosine kinase receptors, such as insulin receptor, growth factor receptors, as well as Src and other cellular oncoproteins.
Steady progress in research on tankyrase subcellular localization shows that our knowledge in this field is still fragmentary. The controversial data obtained in various laboratories are sometimes explained by the usage of different cell cultures. We have assayed the tankyrase activity in several cell types and found the human embryonic kidney cell line 293 to be the best experimental model, since it contains the highest enzyme activity. In the present study we show for the first time that tankyrase is a soluble protein, which can bind to microsomes, cytoskeleton, telomeres, and other subcellular structures, where it should be recruited and involved in poly(ADP-ribosyl)ation of protein acceptors.
MATERIALS AND METHODS
Expression constructs. A construct gfp-H203 for expression of tankyrase-2 fused to GFP in eukaryotic cells was created from plasmid MO-BC-203, a structure of which was recently published [8]. In pBK-CMV vector (Stratagene, USA) the MO-BC-203 contains a 5-kb cDNA insert encoding a large fragment of tankyrase-2 (amino acids 258 to 1166), and also 3´ untranslated region. A part of the latter flanked by HindIII sites was removed, and cDNA of GFP from pGreenLantern (Clontech, USA) was inserted into an SpeI site upstream of N-terminal sequence of the enzyme in the same reading frame. The fused protein GFP-TNKL is comparable in size with native tankyrase-2 (1155 and 1166 amino acids, respectively). A plasmid pGFP for GFP expression in control experiments was obtained by removing of tankyrase cDNA out of gfp-H203 using restriction on EcoRI and XbaI sites, blunting the protruding ends, and recircularization of the shortened plasmid.
Preparations of human cells. Primary cultures of human embryonic and postnatal (12-year-old donor) fibroblasts and myoblasts were from the cell bank of the Institute of Medical Genetics of the Russian Academy of Medical Sciences. They were grown in DMEM [12] containing 10% (v/v) fetal calf serum. Human embryonic kidney cell line 293 and HeLa cell line were grown in the same medium. Tumor cell lines K-562, MOLT-4, and L-41 were kindly provided by E. A. Kalashnikova (Medico-Genetic Scientific Center, Russian Academy of Medical Sciences). Human peripheral blood cells enriched with leukocytes were separated in PBS (Na-phosphate, 7 mM, pH 7.4; NaCl, 150 mM) on a day of experiment. A layer of white cells was isolated after centrifugation at 400g for 10 min.
Transient transfection. A conventional calcium phosphate method was used for transfection of human embryonic kidney cell line 293. A calcium phosphate precipitate was prepared with 1 µg plasmid DNA in 250 µl HBS buffer (HEPES, 6.5 mg/ml; NaCl, 8 mg/ml; Na-phosphate, 0.89 mM; pH 7.0), to which 15.5 µl CaCl2, 2 M, were added. The preparation was incubated 15 min at room temperature and dropped into a 60-mm culture dish with cells, which were grown to log-phase in DMEM with 5% (v/v) fetal calf serum overnight at 20-30% to confluence on a glass cover slip.
Fluorescence microscopy. Preparations were observed using a Nikon Eclipse TE200 microscope (Japan) equipped with Plan Fluor 40× /0.75 objective and pass filters for excitation of FITC (green).
Subcellular fractionation. A cell preparation was washed once with Hanks' balanced salt solution [12], twice with ice-cold TBS (Tris/HCl, 20 mM, pH 7.4; NaCl, 150 mM), and was scraped in 5 ml of ice-cold buffer F (Tris/HCl, 11 mM; sucrose, 275 mM; EDTA, 0.55 mM, pH 7.4) [4, 9]. Cells were homogenized in a tight-fitting glass-Teflon homogenizer and clarified by centrifugation at 1500g for 5 min at 4°C. The pellet was discarded, and the supernatant was centrifuged again at 50,000g for 20 min at 4°C to remove microsomes of high density. The pellet, which did not contain tankyrase (data not shown), was discarded. The supernatant was used for the tankyrase enzyme assay. If necessary, a fraction of low-density microsomes was recovered by centrifugation at 160,000g for 70 min at 4°C. The pellet was resuspended in 5 ml buffer F after separation of the supernatant, which consists of soluble substances.
Antipeptide antibodies. A synthetic oligopeptide H2N-NGVRSPGATADA-COOH was used to raise polyclonal anti-tankyrase rabbit antiserum. Its structure corresponds to a unique region in the amino acid sequence of tankyrase-2 (amino acids 813-824), which was confirmed by analysis of Genbank database using Protein BLAST software, Search for short nearly exact matches (http://www.ncbi.nlm.nih.gov/BLAST/). For immunization 0.2 µmol of the peptide was conjugated to 1 mg of bovine serum albumin, a carrier protein, using glutaraldehyde. A complete characterization of the antibodies will be published elsewhere.
Enzyme activity assay. To assay the tankyrase enzyme activity, the method of autocatalytic poly(ADP-ribosyl)ation was used [1, 2, 4, 11]. The enzyme preparation was incubated at room temperature 2-40 min (routinely 30 min) in reaction buffer containing Tris/HCl, 50 mM, pH 8; MgSO4, 4 mM; [32P]NAD+, 10 µCi/ml, 1 µM. The preparation of [32P]NAD+ was synthesized and kindly provided by Yu. S. Skoblov (Engelhardt Institute of Molecular Biology, Russian Academy of Sciences). The inhibitor niacinamide (Sigma, USA), 10 mM, was added into a parallel sample, which was used as a negative control. When incubation was completed, the reaction was stopped with ice-cold acetone mixed in a ratio 1 : 1 (v/v). The protein was precipitated during incubation 20 min at -18°C and following centrifugation at 3000g for 5 min. The supernatant was discarded, and the pellet was resuspended in sample buffer (Tris/HCl, 125 mM, pH 6.8; SDS, 1 mg/ml; glycerol, 10% (v/v); 2-mercaptoethanol, 1% (v/v); bromophenol blue, 0.01 mg/ml), boiled for 3 min, and loaded onto 6% polyacrylamide gel [13].
Electrophoresis under denaturing conditions (SDS-PAGE) was performed at room temperature in standard Tris/glycine buffer (Tris, 3.02 g/liter; glycine, 14.5 g/liter; SDS, 1 g/liter; pH 8.3) at 100 V in a 1 × 80 × 100 mm gel. Rainbow labeled markers (Amersham Pharmacia Biotech, Sweden) and Molecular Weight Marker Kit (ICN Biomedicals, Inc., USA) were used as markers of molecular mass. When SDS-PAGE was completed, the gel was stained with Coomassie blue G-250 (ICN Biomedicals, Inc.), exposed to X-ray film, and radioactive labeling of protein was detected.
Western blots. To visualize tankyrase-2 using antipeptide polyclonal antiserum, the protein was transferred from SDS-PAGE gel to nitrocellulose Hybond-C (Amersham Pharmacia Biotech) within a semi-dry transfer cell Enprotech (Integrated Separation Systems, USA) at 200 mA for 45 min. The transfer buffer contained Tris, 5.8 g/liter; glycine, 2.9 g/liter; SDS, 0.385 g/liter; ethanol, 40% (v/v). The nitrocellulose membrane was then blocked with 1% (w/v) ovalbumin in PBS, incubated with the antipeptide polyclonal antiserum diluted in PBS 1 : 20, vigorously washed with PBS with 0.1% (v/v) Triton X-100, incubated with anti-rabbit second antibodies labeled with horseradish peroxidase (Clontech), washed again, incubated with peroxidase substrates Biowest (UVP, Inc., USA), and exposed to X-ray film for signal detection.
Gel filtration. The tankyrase preparation from subcellular fraction of soluble proteins of human embryonic kidney cell line 293 was incubated 5 min in the reaction buffer for tankyrase enzyme assay (see above). When incubation was completed, 0.3 ml of the reaction mixture was loaded onto a column (700 × 10 mm) with Sepharose 2B (Amersham Pharmacia Biotech) equilibrated with buffer S (Tris/HCl, 20 mM; KCl, 150 mM; pH 8). The column was calibrated with bovine serum albumin (ICN Biomedicals, Inc.). Fractions, 1 ml each, were eluted with buffer S at 4°C, and their radioactivity was monitored using a Tracor Analytic Delta 300 scintillation counter (ThermoQuest/CE Instruments, USA). In aliquots protein was assayed [14, 15] and precipitated with ice-cold acetone added at ratio 1 : 1 (v/v) as mentioned above (see “Enzyme activity assay”). The pellet of each aliquot was resuspended in the sample buffer and used partly for SDS-PAGE and partly for radioactivity detection using the scintillation counter.
RESULTS
Expression of recombinant tankyrase-2 in culture of human embryonic kidney cell line 293. To study the subcellular localization of tankyrase-2, we have created the expression construct gfp-H203. Its transfection into human cells leads to a synthesis of the enzyme with an additional N-terminal protein domain of GFP (Fig. 1). The fluorescence of the latter provides an advantage of easy detection of the chimeric protein in living cells.
As shown in Fig. 2, in interphase the fusion protein was excluded from nuclei, while in control culture the expression of GFP by itself was followed by its accumulation in the whole transfected cell including the nucleus. Indeed, such a massive protein as tankyrase-2 cannot passively diffuse through nuclear pores as GFP does. Without special transfer proteins the enzyme must be localized within the extranuclear space.Fig. 1. Structure of tankyrase-2 and fused protein GFP-TNKL constructed for its subcellular localization in vivo. a) Amino acid sequence of tankyrase-2. Ankyrin repeats, SAM, and PARP domains are designated by text, underlined are positions 813-824 corresponding to the synthetic peptide that was used to raise anti-tankyrase-2 polyclonal antiserum. b) Domain structure of tankyrase-2 (TNKL) and a fused protein GFP-TNKL. Ankyrin and other domains are designated by text.
Identification of endogenous tankyrase in subcellular fractions. Our next step was the identification of endogenous tankyrase in subcellular fractions. For that purpose, we raised polyclonal antiserum against synthetic oligopeptide whose structure corresponds to one of the unique regions in the amino acid sequence of tankyrase-2. In the ankyrin domain of TNKL there are five clusters of three highly conservative ankyrin repeats connected to each other by less conserved repeats with irregular insertions and deletions. To obtain antibodies specific to tankyrase-2, we chose as an antigen a short sequence from one of the less conserved regions (Fig. 1).Fig. 2. Subcellular localization of tankyrase-2 fused to GFP. Fluorescence of GFP-TNKL chimeric protein was observed in only the extranuclear space of transfected cells (d). In control preparation the free GFP was seen in both nuclei and cytoplasm of the plasmid containing cells (c). a, b) Phase contrast slides of the same cells; the medium contains the Ca-phosphate precipitate (see “Materials and Methods”).
With these antibodies a protein of expected molecular mass (about 130 kD) and several polypeptides of smaller size were detected in lysates and subcellular fractions of human embryonic kidney cell line 293 (Fig. 3a, left panel). Most of the enzyme was found in low-density microsomes and the soluble protein fraction. Accumulation of the lower bands was observed generally in microsomal fraction, especially during storage of concentrated preparations, which can be explained by the proteolytic degradation of tankyrase.
Using the enzyme assay to detect tankyrase, we have found it in the same subcellular fractions (Fig. 3a, right panel). A radioactive labeling of mostly 130-kD polypeptide was detected in presence of [32P]NAD+. Boiling of the enzyme preparation completely prevented the protein labeling (data not shown), suggesting that the radioactive product of high molecular mass was a result of enzymatic activity. A specific inhibitor of NAD+-ADP-ribosyltransferases, niacinamide, drastically decreased the quantity of the radioactive protein (Fig. 3a, right panel, lane 3). The tankyrases, two proteins out of six enzymes in the human genome known as poly(ADP-ribosyl)polymerases with autocatalytic activity [16], have distinct molecular masses, 142 and 127 kD in non-modified forms. From data shown in Fig. 3a we conclude that one or both tankyrases were visualized under the experimental conditions.Fig. 3. Assays of endogenous tankyrase in subcellular fractions. a) Visualization of endogenous tankyrase in human embryonic kidney cell line 293 using antipeptide antibodies and poly(ADP-ribosyl)ation. Subcellular fractions of the soluble proteins (1) and low-density microsomes (2) after SDS-PAGE and Western blotting using anti-tankyrase-2 antiserum contained a 130-kD protein that reacts with the antibodies (left panel). Visualized lower molecular mass species were accumulated in 10-time concentrated microsomal preparation (lane 3, left panel) and presumably produced by proteolytic degradation. In parallel the same preparations were incubated with [32P]NAD+, 1 µM, and poly(ADP-ribosyl)ation of the same 130-kD protein and its fragments was detected (right panel). Lane 3 (right panel) represents a negative control; the enzyme reaction was carried out in the presence of inhibitor, niacinamide, 10 mM. b) Time course of the enzymatic reaction. Aliquots of the soluble fraction were used to detect poly(ADP-ribosyl)ation of tankyrase. The reaction was stopped after 2, 4, 6, or 40 min of incubation (lane S). Left, protein staining with Coomassie; right, autoradiography of the same gel (see “Materials and Methods”). The kinetics of the poly(ADP-ribosyl)ation of microsomal tankyrase (lane M) was not observed under the experimental conditions. c) Poly(ADP-ribosyl)ation of the enzyme preparations of the combined soluble and microsomal fractions from various types of human cells under standard conditions and similar protein concentration. The following cells were used: embryonic kidney cell line 293 (1), embryonic (2) and postnatal (3) fibroblasts, myoblasts (4), peripheral blood leukocytes (5), erythroleukemia K-562 cell line (6), lymphoblastic leukemia MOLT-4 cell line (7), myeloleukemia L-41 cell line (8), cervical adenocarcinoma HeLa cell line (9). Left, protein staining with Coomassie; right, autoradiography of the same gel (see “Materials and Methods”).
Besides the mentioned cytosolic and microsomal fractions, the tankyrase was also observed in the fraction of nuclei and large cell debris (data not shown). With these experimental conditions we could not determine whether the enzyme might also be located in nuclei, or that the fraction had an impurity of soluble and/or microsomal tankyrase.
Solubility of tankyrase from human embryonic kidney cell line 293. Both tankyrases have already been observed in microsomal fraction of some cells [4, 9]. Sometimes a small part of the enzyme was also detected in soluble fraction, but this pool did not attract attention. To find out whether the soluble tankyrase was not an artifact of fractionation, we studied kinetics of the enzyme reaction and molecular mass of the enzyme in cytosolic fraction.
Figure 3b presents a time course of accumulation of the protein labeled in the presence of [32P]NAD+. The radioactivity was generally associated with the 130-kD polypeptide. At constant protein concentration, specific radioactivity elevated with time of incubation until it reached a plateau at more than 6 min, as should be expected for enzyme kinetics. Simultaneously a decrease of electrophoretic mobility was observed, which is a characteristic feature of species bearing covalently bound poly(ADP-ribose) of heterogeneous length. Interestingly, no significant polymerization of ADP-ribose was seen in freshly prepared microsomal fraction (during storage the protein may dissociate out of microsomes, data not shown). Therefore, in the reaction of poly(ADP-ribosyl)ation the soluble tankyrase from human embryonic kidney cell line 293 exhibits all described characteristic properties of tankyrase [1, 2, 4, 11].
Applying the enzyme activity assay to the protein from different sources, we have found that under identical conditions our model embryonic kidney cell line 293 had maximal amount of enzyme (Fig. 3c). Several cancer cell lines also contained some activity, while normal peripheral leukocytes, myoblasts, embryonic and postnatal fibroblasts had no detectable tankyrase. A high level of the enzyme activity in our model cell line may therefore provide a possible explanation for the significant concentration of free soluble enzyme under our experimental conditions comparatively to published data. In other cells the protein may be mostly bound.
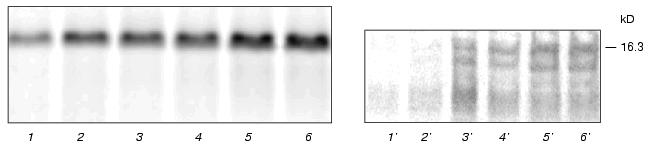
Figure 4 presents an elution profile of the soluble ADP-ribosylated enzyme during gel filtration on Sepharose 2B. In extracts free from microsomes (the microsomal tankyrase was sorbed within the column, data not shown) more than 90% of the labeled modified protein from 293 cell line is the 130-kD polypeptide, which is presumably tankyrase. It was found in fractions corresponded to elution of macromolecules of molecular mass no more than 150 kD (Fig. 4a), seemingly the mass of monomeric tankyrase. In the same fractions tankyrase-2 was detected (Fig. 4b) with specific antiserum. No large protein aggregates and/or organelle-borne tankyrase were found by means of size exclusion chromatography. Therefore, under the experimental conditions, the endogenous ADP-ribosylated tankyrase-2 from human embryonic kidney cell line 293 was eluted as a protein free from association with membranes or other large proteins.
Fig. 4. Size exclusion chromatography of soluble fraction from lysate of human embryonic kidney cell line 293. The cell lysate was filtered to remove membrane fractions, incubated with [32P]NAD+, and loaded onto the Sepharose 2B column (70 × 1 cm). a) Elution profile of protein (curve 1) and radioactivity. The arrow indicates the elution peak of bovine serum albumin (136 kD). The total radioactivity (curve 2) was detected in aliquots using a scintillation counter. To determine the protein radioactivity (curve 3), another aliquot of each fraction was mixed with acetone 1 : 1, the protein was precipitated, resuspended in a fresh portion of buffer, and moved to a scintillation counter vial. b) Coomassie stain (1), autoradiography (2), and Western blot using anti-tankyrase-2 antiserum (3) of polyacrylamide gel/nitrocellulose blot after SDS-PAGE of protein containing fractions. The protein was precipitated with acetone, resuspended in Laemmli sample buffer, and loaded onto polyacrylamide gels, one of which was used for Coomassie staining and autoradiography, and a parallel one was used for Western blotting. Tankyrase-2 was visualized by both antibodies and the enzyme assay methods in the same fractions.
DISCUSSION
It is well known that the proteins implicated in cell signaling, e.g., receptors, may be normally expressed at a very low level. This feature produces difficulties for their studies, such as subcellular localization of signaling proteins. Tankyrase, which was initially detected on telomeres [1], was then shown on nuclear membrane [3], in Golgi [4], extranuclear membrane vesicles [4, 9], in cortical actin cytoskeleton [5], and in mitotic apparatus [3, 6].
In this report we have shown that tankyrase can also be soluble in the aqueous phase. The study of such phenomenon has become possible using human embryonic kidney cell line 293, where a significant pool of the protein (up to 50%) was located in the soluble fraction. In general the 293 cell line is an extremely rich source of tankyrase (Fig. 3c), seemingly having a high activity of the signaling pathway with participation of the enzyme under study.
The recombinant tankyrase-2 fused to GFP for its in vivo detection was visualized in the same model cell line (Fig. 2). In contrast to free GFP in control preparation, the fluorescent protein fused to tankyrase was excluded out of the cell nuclei. These data confirm previously published observations [4, 9] and mean that the overexpressed tankyrase in 293 cell line is mostly concentrated in extranuclear compartments. On the other hand, the limited sensitivity of our detection method cannot allow ruling out the binding of a minute amount of tankyrase in nuclei, as was observed by other methods [1, 3].
Since the additional N-terminal GFP domain in recombinant tankyrase could interfere with the intracellular traffic of the chimeric protein, in subsequent studies we used more independent methods. The endogenous enzyme was detected using two approaches, namely visualization by tankyrase-2 specific antibodies on immunoblots and autocatalytic radioactive labeling in [32P]NAD+-containing buffer in vitro. From our data one can conclude that a significant amount of tankyrase was found in the fraction of soluble proteins. About only a half of the enzyme was located in microsomal fraction (Fig. 3a). The enzymatic properties of the soluble tankyrase from 293 cell line (Fig. 3b) were close to those described by researchers using the baculovirus-expressed human enzyme [1, 2], the chimeric human enzyme from BOSC cells [11], and the endogenous enzyme from murine 3T3 fibroblasts [4]. Although only membrane, chromosome, or cytoskeleton bound tankyrase was observed this far, the solubility of a significant enzyme pool can probably be explained by its greater activity in our model cell line (Fig. 3c).
To rule out the possibility that the soluble fraction after ultracentrifugation still contains impurities of microsomal or actin-bound tankyrase, we estimated the molecular mass of the enzyme using size exclusion chromatography (Fig. 4). Under the experimental conditions the ADP-ribosylated enzyme was eluted from the Sepharose 2B column as a monomer free from both microsomes and other large complexed proteins, as could be expected taking into account previous publications [2, 5, 9, 10]. The protein under study was identified by two independent methods, the enzyme assay and immunoblotting. The latter allows also the determination that the eluate contained the isoenzyme tankyrase-2.
On the basis of the described evidence, we suggest that the known association of tankyrase with various subcellular compartments [4, 5, 9, 11] may be a result of interaction of the soluble enzyme with its substrates for posttranslational modification and other proteins of microsomes, nuclear membrane, cytoskeleton, etc.
The very existence of the soluble tankyrase may be a basis for one more suggestion, the verification of which could be useful to learn more about the function of this signaling molecule. The soluble protein may be the active form of tankyrase, dangerous and therefore rare in living cells. Using intracellular NAD+, it transduces the regulatory signal into different compartments. As Chi and Lodish have shown [4], the microsomal tankyrase can be phosphorylated with MAP kinase, which apparently enhances its activity. The telomeric tankyrase also needs activation [2]. The microsomes might be storage for the inactive enzyme, and after the activation tankyrase would dissociate into cytosol and be transferred toward the nucleus and plasma membrane, where it interacts with the cortical actin cytoskeleton [5]. It is also noteworthy that we have found different tankyrase activity in various cell types (Fig. 3c), while it is known that all studied organs and tissues have a TNKL transcript [2, 8, 10]. To solve this discrepancy in subsequent research it will be necessary to study the possibility of regulation of TNKL synthesis at the translation step and to test other hypotheses about tankyrase activation.
The authors highly appreciate help by I. S. Grigoriev (School of Biology, Lomonosov Moscow State University) for assistance in microscopy, N. S. Egorova and E. A. Sukhacheva (Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences) for oligopeptide synthesis and raising polyclonal antiserum, Yu. S. Skoblov (Engelhardt Institute of Molecular Biology, Russian Academy of Sciences) for [32P]NAD+ synthesis, E. A. Kalashnikova (Medico-Genetic Scientific Center, Russian Academy of Medical Sciences) for cultures of cancer cell lines, N. S. Filippov (Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University) for assistance in ultracentrifugation, D. Kuprash (Engelhardt Institute of Molecular Biology, Russian Academy of Sciences) for assistance in creating of expression constructs and valuable discussion, and S. A. Nedospasov (Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, and Engelhardt Institute of Molecular Biology, Russian Academy of Sciences) for support in organization of the scientific research, valuable discussion, and participation in the manuscript preparation.
This study was supported by the Russian Foundation for Basic Research (grant No. 00-04-48300, principal investigator A. N. Kuimov), the plasmid MO-BC-203 used for creating of expression constructs was obtained with financial support of the Ludwig Institute for Cancer Research (project “Identification and Characterization of Novel Cancer Antigens”, principal investigator S. A. Nedospasov).
REFERENCES
1.Smith, S., Giriat, I., Schmitt, A., and de Lange,
T. (1998) Science, 282, 1484-1487.
2.Cook, B. D., Dynek, J. N., Chang, W., Shostak, G.,
and Smith, S. (2002) Mol. Cell Biol., 22, 332-342.
3.Smith, S., and de Lange, T. (1999) J. Cell
Sci., 112, 3649-3656.
4.Chi, N.-W., and Lodish, H. F. (2000) J. Biol.
Chem., 275, 38437-38444.
5.Seimiya, H., and Smith, S. (2002) J. Biol.
Chem., 277, 14116-14126.
6.Sbodio, J. I., and Chi, N.-W. (2002) J. Biol.
Chem., 277, 31887-331892.
7.Monz, D., Munnia, A., Comtesse, N., Fischer, U.,
Steudel, W. I., Feiden, W., Glass, B., and Meese, E. U. (2001) Clin.
Cancer Res., 7, 113-119.
8.Kuimov, A. N., Kuprash, D. V., Petrov, V. N.,
Vdovichenko, K. K., Scanlan, M. J., Jongeneel, C. V., Lagarkova, M. A.,
and Nedospasov, S. A. (2001) Genes Immunol., 2,
52-55.
9.Lyons, R. J., Deane, R., Lynch, D. K., Ye, Z. S.,
Sanderson, G. M., Eyre, H. J., Sutherland, G. R., and Daly, R. J.
(2001) J. Biol. Chem., 276, 17172-17180.
10.Kaminker, P. G., Kim, S. H., Taylor, R. D.,
Zebarjadian, Y., Funk, W. D., Morin, G. B., Yaswen, P., and Campisi, J.
(2001) J. Biol. Chem., 276, 35891-35899.
11.Sbodio, J. I., Lodish, H. F., and Chi, N.-W.
(2002) Biochem. J., 361, 451-459.
12.Ham, R. G., and McKeehan, W. L. (1979) Meth.
Enzymol., 58, 44-93.
13.Laemmli, U. K. (1970) Nature, 227,
680-685.
14.Bradford, M. M. (1976) Analyt. Biochem.,
72, 248-254.
15.Wilson, C. M. (1983) Meth. Enzymol.,
91, 236-247.
16.Smith, S. (2001) Trends Biochem. Sci.,
26, 174-179.