Occupancy of Two Primary Chloride-Binding Sites in Natronobacterium pharaonis Halorhodopsin Is a Necessary Condition for Active Anion Transport
I. V. Kalaidzidis and Ya. L. Kalaidzidis*
Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3181; E-mail: yannis@phtbio.genebee.msu.su* To whom correspondence should be addressed.
Received December 6, 2002
The existence of two primary chloride-binding sites was found on the basis of the study of halorhodopsin spectra at different chloride concentrations. SVD analysis of the spectra revealed two chloride-dependent components at low chloride concentration (0.1-10 mM). Global fitting of SVD components found KD values of 0.47 and 5.2 mM with unitity Hill coefficients. The second KD coincides with the apparent KD of the photovoltage response of halorhodopsin.
KEY WORDS: halorhodopsin, chloride-binding site, halorhodopsin electrogenesis, Natronobacterium pharaonis, SVD analysis
Abbreviations: hR) halorhodopsin; phR) Natronobacterium pharaonis halorhodopsin; shR) Halobacterium salinarum halorhodopsin; SVD) singular value decomposition.
Halorhodopsin (hR) is a light-dependent chloride pump of cytoplasmic
membranes of halobacteria. Halorhodopsin shares certain similarity in
amino acid sequence, three-dimension structure, and functioning with
bacteriorhodopsin, a proton pump of halobacteria. Like
bacteriorhodopsin, hR contains protonated aldimine of retinal as a
chromophore. Retinal photoisomerization
(all-trans-->13-cis) triggers a photocycle,
representing sequential protein rearrangements which correspond to
spectral intermediates observed during optical measurements. However,
these proteins differ in ion specificity and transport direction. The
photocycle of hR is accompanied by chloride-ion transfer into the
cytoplasm. Transmembrane transfer of chloride-anion is accompanied by
generation of electric potential difference on the membrane and its
measurement can be used to determine intraprotein charge transfer
during various stages of the photocycle [1].
In contrast to well-studied bacteriorhodopsin, many details of hR structure and functioning still require further investigation. Mechanism and the route of Cl- transport inside this protein remain unknown, information on its photocycle is quite contradictory, and the number of chloride-binding sites still represents a matter for discussion.
Studies of hR functioning as a chloride pump usually use one of two of these retinal-containing proteins: Halobacterium salinarum halorhodopsin (shR; 271 amino acid residues) and Natronobacterium pharaonis halorhodopsin (phR; 285 amino acid residues). These proteins share 65% identity. Although phR structure with high resolution has not been obtained yet, it is believed that these proteins have similar structure and only quantitative differences between the two exist. (For example, phR might have a wider channel for anion transfer, and this can be used to explain effective transfer of nitrate and some other anions.)
Since phR demonstrates chloride-dependent photocycle kinetics (and some other characteristic features) it is considered as a more convenient and useful model system [2, 3]. Analysis of spectral kinetics revealed that decay of the O-intermediate is the most chloride-dependent reaction. It led to the following scheme of the photocycle for chloride-binding form of phR: HR --> K <--> L <--> N <--> O <--> HR´ --> HR. Chloride release and uptake were attributed to N --> O and O --> HR´ stages, respectively. All dark stages (except the last one) are reversible. Chizhov and Engelhard [4] proposed the following scheme of the photocycle HR --> K --> L1 --> L2 --> LO --> ON --> HR´ --> HR, which is only partially comparable by intermediates proposed earlier [3]. The essential difference of this scheme is irreversibility of all dark reactions.
Results of our recent study of electrogenesis [1] suggest the necessity of revision of this scheme because electrogenesis did not reveal any component similar to decay components of the O-intermediate. We also found that the photoelectric response of phR in the presence of chloride anion consists of two main components. The amplitude of the fast component (tau ~ 1 µsec) represented ~4% of the whole signal, whereas the rest of this signal belonged to a single exponential slow component of tau ~ 1 msec. Neither time constant exhibited significant chloride-dependence.
Although in the introduction of the paper [5] the authors questioned our data, actual results of that study of photoelectric responses indicated lack of the electrogenic component coupled to decay of O-intermediate. At chloride concentrations up to 2 M photoelectric responses of shR also did not demonstrate the presence of chloride-dependent components [2].
It should be noted that two scientific schools give rather distinct definition of the term “electrogenesis”. We belong to the school which considers electrogenesis as appearance of membrane potential as the result of transition of distinct protein states (e.g., O-intermediate decay). Our opponents [5] belong to another school which interprets “electrogenesis” as a difference of dipole moments of corresponding protein state and the main state (before the beginning of the cycle). Thus, the conclusion made by these authors [5] that electrogenesis of the O-intermediate is identical to electrogenesis of HR´-intermediate means lack of changes in the membrane potential during the O <--> HR´ transition. (The latter is consistent with our own data.)
Since within the scheme HR --> K <--> L1 <--> L2 <--> N <--> O <--> HR´ --> HR [3, 5] decay of N- and formation of O-intermediate are the same reaction, we may say that the corresponding component of electrogenesis equally belongs to both decay of N- and formation of O-intermediate. So attribution of the main stages of chloride transfer to O-intermediate requires to be confirmed and our hypothesis [1] that the electrogenesis is coupled to L- and N-intermediate and O-intermediate is related to a side-cycle is still valid. Note that our viewpoint is also supported by Muneyuki et al. [6].
Otomo [7] hypothesized the existence of a chloride-binding site on the surface of shR located near the loop connecting alpha-helices B and C. This is based on the observation that point mutation of His95 located within this loop is critical for active transport of chloride-anion. Until that report, it was believed that hR molecule contains only one chloride-anion binding site related to the transport and located in the inner region of this protein near the Schiff base. However, that study did not receive further development.
It should be noted that shR contains a chloride-binding site near the beta-ionone ring of retinal, known as “site II” [8]. Chloride binding at this site is accompanied by a red-shift of the maximum of the hR absorbance spectrum. However, it is generally accepted that this site is not involved into chloride transport. The existence of a similar site in phR has not been found.
The authors of works [2, 5] defined the KD value for the chloride binding site near the Schiff base equal to 1 mM. The titration curve was well approximated to the theoretic one with Hill coefficient of 0.75. However, the reason for deviation of Hill coefficient from 1 was not explained.
However, Using concentration-dependence of the photoelectric response of phR the KD value was determined to be 5-6 mM, with Hill coefficient of 1 [1, 9].
Thus, there is evident inconsistency between KD values obtained during studies of hR electrogenesis and spectral changes. This study has been undertaken to solve this problem.
We hypothesized that reports on various KD values and non-unity Hill coefficient reflect the existence of more than one chloride-anion binding site. So we accurately measured phR spectra at different NaCl concentration and subjected the data obtained to mathematical treatment.
MATERIALS AND METHODS
Freshly isolated preparations of hR from H. salinarum L33 strain transformed using vector containing structural hop gene from N. pharaonis and lacking its own genes encoding hR and bacteriorhodopsin were used in the study. This strain was kindly given to us by Professors J. Lanyi and R. Needleman. Cultivation procedure and isolation of fragments of cell membrane were carried out as described in [2].
All experiments were carried out using dark-adapted preparations at 10 µM hR. Absorbance spectra were recorded using a Hitachi-3400 spectrophotometer (Japan) at the maximal rate of scanning (5 nm/sec). Experimental curves were obtained within the wavelength range from 330 to 800 nm (using 0.5 nm interval). All set of results obtained as plots of DeltaA versus lambda was transformed into three dimensional “surface” ([Cl-], lambda, DeltaA). For analysis of the experimental data we employed GraphView from PLUK software (Ya. L. Kalaidzidis) developed on the basis of PLUK language [10]. This software is available at the following address: http://www.med.mics.msu.su/pluk/default.html. Errors of decomposition parameters were evaluated by elements of principal diagonal of reversed Hessian chi2.
RESULTS AND DISCUSSION
For determination of number of chloride binding sites and their KD values we investigated the effect of NaCl titration on the behavior of phR spectrum during the transition of chloride-free phR589 form into chloride-bound form.
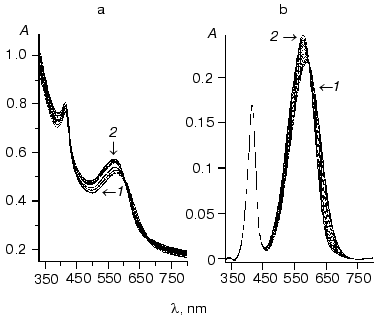
Due to strong light-scattering on membranes (Fig. 1a) it was nearly impossible to determine correctly the position of maximal absorbance and spectrum shape. So, initial spectra were corrected for membrane light-scattering before plotting the dependence of the phR spectrum on chloride concentration. Analysis of the resultant spectra within the range of wavelength 300-700 nm did not reveal the presence of two distant regions lacking “wing lines” suitable for direct recognition of the light scattering function. To find light scattering we employed decomposition of a spectrum into three components: two asymmetric lines described by log-normal curves and light scattering, similarly to the method used by Chizhov and Engelhard [4]:
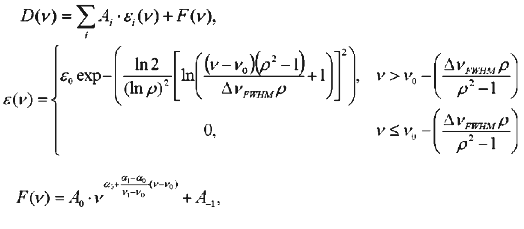
where D(nu) is the observed spectrum, epsilon(nu) the log-normal distribution, epsilon0 the molar absorbance coefficient at the wavelength 1/nu0, nu0 the frequency of absorbance maximum, nu the frequency, DeltanuFWHM the full width of the line at half-height, rho the asymmetry of spectrum determined as a ratio of right to left semi-width of spectrum at the half-height, F(nu) the light scattering, and coefficients Ai are weight contribution of components into the total spectrum.
Chizhov and Engelhard [4] considered alpha as a wavelength-independent parameter. However, in the case of our experiments (within the spectral range 330-800 nm) the ratio of wavelength to the size of a dissipating particle which determines alpha value changed dramatically. So, for approximation of alpha(nu) we used the following (first) order of decomposition of the function into Taylor's series. Decomposition was carried out using GraphView.Fig. 1. A set of spectra obtained during titration of phR589 with NaCl: a) initial spectra; b) the same spectra corrected for light scattering and normalized with respect to peak with lambdamax of 415 nm. Concentration of chloride anions was varied from 10 µM to 1 M. The assay medium also contained 0.5 M Na2SO4, 5 mM MES, pH 6.4: 1) chloride-free form; 2) 1 M NaCl.
One log-normal decomposition component had a maximum at 415.6 nm, another one was characterized by the maximum within 575-590 nm and was individually determined for each spectrum. Evidently, description of hR spectrum at intermediate values of chloride concentrations by a single log-normal component with the maximum within the region of 575-590 nm is very approximate because the spectrum contains at least two bands, chloride-free and chloride-binding. However, this approximation was sufficient for determination and subsequent removal of light-scattering function.
The resultant spectra were normalized by peak amplitude with maximum of 415 nm belonged to a cytochrome present in red membranes (see Fig. 1b).
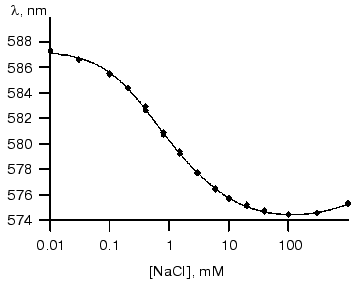
Figure 2 shows the resultant dependence of absorbance maximum on chloride concentration. The shape of this titration curve shows that it describes more than one process because it has one minimum followed by augmentation at the higher salt concentration.
This curve can be well described by two S-shaped dependencies with KD of about 2 M and Hill coefficient close to unity for an increase at high NaCl concentrations and KD of 0.8 ± 0.02 mM and Hill coefficient significantly differed from unity (0.73 ± 0.02) for the decline. This is consistent with literature data (KD = 1 mM and Hill coefficient = 0.75) [2].Fig. 2. Dependence of absorbance maximum of phR589 on chloride concentrations (dots). Solid line shows fitting with KD1 of 0.43 mM, KD2 of 3.9 mM, and KD3 of 3 M.
Interestingly, the curve behavior of dependence of shift of spectrum maximum on chloride concentration can explain the difference between maximal absorbance wavelength attributed to chloride-bound phR observed in [4] (574 nm) and [2] (577 nm) because authors determined lambdamax at strongly differed NaCl concentrations (0.1 and 2 M, respectively).
The value of Hill coefficient less than unity suggests the existence of either negative cooperativity of unclear nature or two sites with similar KD values.
For determination of the actual number of chloride dependencies and consequently number of chloride binding sites we subjected the set of titration spectra to SVD analysis [11].
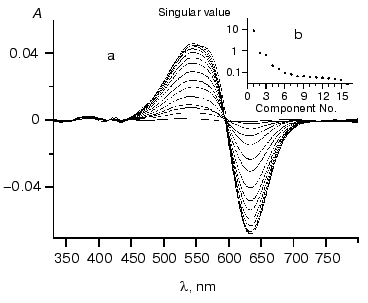
To reduce the effect of noise on the result of SVD decomposition we analyzed differential spectra [12] obtained by subtracting the chloride-free spectrum (Fig. 3a). Figure 3b shows the resultant singular values.
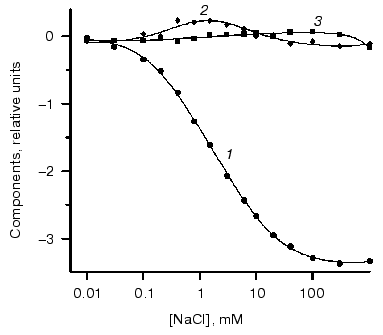
On the basis of analysis of singular value we can suggest the existence of three significant components. Figure 4 shows components of chloride dependencies corresponding to the first three singular values.Fig. 3. a) Differential spectra of phR589 titration by NaCl. After correction of light scattering the resultant spectra were obtained by deduction of spectrum of chloride-free form. Chloride concentration varied from 10 µM to 1 M. Other conditions: 0.5 M Na2SO4, 5 mM MES, pH 6.4. b) Singular values of SVD decomposition of the differential spectra.
It should be noted that the bell-shaped second component (a combination of two sigmoid dependences) unquestionably indicates the presence of two closely positioned KD in the range of 0.1-10 mM. So we carried out global fitting of all three components to three theoretical curves. The following values of KD (0.47 ± 0.15 mM, 5.2 ± 1.7 mM, and 2.5 ± 1.5 M) and corresponding values of Hill coefficient (0.91 ± 0.08, 1.04 ± 0.12, and 1.6 ± 0.2) have been obtained.Fig. 4. SVD components corresponding to the first three singular values obtained during decomposition of spectra of Fig. 3a. Dots represent decomposition, and solid line shows the result of global fitting to three Henderson-Hasselbach curves with the following parameters: KD1 = 0.43 mM, KD2 = 4.7 mM, KD3 = 2.5 M. Components are numbered in the reducing order of corresponding singular values.
It is noteworthy that the curve of the dependence of maximum position at hR absorbance spectrum on chloride concentrations was rather close to the first SVD component. To compare it with the SVD analysis results, we decomposed the curve of maximum position into three components, which also gave very similar results of KD values: KD1 = 0.5 mM, KD2 = 4.7 mM, and KD3 = 3 M. This also supports the results shown above.
Figure 2 shows that occupancy of the binding site characterized by KD value of 2-3 M was accompanied by a red shift of the hR spectrum. Such behavior is consistent with that of shR observed during occupancy of site II [8] localized in the vicinity of the beta-ionone ring of the retinal. Since site II of shR binds only anions of small radii, it is possible that such high KD value for the similar site in phR is determined by steric reasons. Discovery of site II analog in phR eliminates differences in the behavior of two hRs indicated by some authors [2].
The existence of two binding sites at low chloride concentrations can explain the difference between KD value obtained during absorbance maximum titration and amplitude of electrogenesis. The KD value of ~5 mM for the binding site is in line with results of electrogenesis study [1]. To exclude misunderstanding with site II of H. salinarum halorhodopsin, this site with KD of ~5 mM we denominate as site III.
It should be noted that chloride dependence of the photoelectric response is characterized by KD of ~6 mM and Hill coefficient of unity. This suggests that the cycle of chloride transfer begins only after occupancy of both sites, I and III.
In the case of two main binding sites the presence of a single msec component (one tau) of rise in electrogenesis may suggest that there are coupling reactions of anion transfer or one of two anions is not directly involved in transport.
Discrimination between these two possibilities allows analysis of previously obtained results [1] of kinetic measurements of the photoelectric response during application of electric field inhibiting chloride transfer due to generation of diffuse potential on the liposomes membrane. For this purpose a medium in the compartments of the electro-measurement cuvette containing 10 mM NaCl was replaced (using a peristaltic pump) for medium containing 1 M NaCl. It resulted in three-exponential increase in potential characterized by 1.2 msec (50%), 5 msec (16%), and 26 msec (34%). Subsequent incubation restored a single-exponential kinetics with tau ~ 1 msec. If the initial medium contained 0.5 M Na2SO4 corresponding attenuation was not observed.
We interpret this effect as an indication for separation of coupling reactions of two chloride transfers during application of the inhibiting field due to generation potential by sodium ions in the process of their diffusion into the inner space of liposomes.
Employment of flash spectroscopy revealed that formation and decay of the O-intermediate are chloride-dependent stages of the phR photocycle, which were suggested to be responsible for release and uptake of chloride anion [2, 3]. According to literature data, formation and decay of the O-intermediate are related to disappearance/appearance of chloride near the Schiff base and therefore one could expect that due to significant movements of chloride these reactions should make the main contribution to electrogenesis. However, our measurements did not reveal any chloride-dependent component(s) with time intervals typical for the O-intermediate decay characterized by chloride uptake as proposed by [1]. On the basis of our data we conclude that the O-intermediate decay (chloride binding) is a non-electrogenic process. This fact together with data on phR titration with NaCl suggests the existence of a surface binding site. (Existence of such site was hypothesized in [5, 7].) It is important to stress that the surface localization of the binding site revealed in the present study well explains lack of chloride dependence of the photoelectric response main time component (tau).
Since the site with KD of 0.47 mM is the “main contributor” to the spectral shift, hence, it can be concluded that it is positioned closer to the Schiff base, whereas KD of 5.2 mM corresponds to the surface site. The existence of weak effect of this surface binding site on the hR spectrum we explain by electrostatic interaction between anions of both sites localized on the surface and near the Schiff base. Such effect of charge interactions between the binding sites localized on the surface and near the Schiff base was also observed in the case of bacteriorhodopsin [13].
Results of the present report provide convincing evidence for involvement of two chloride anions bound to hR under dark conditions in the active transport of chloride. Since half of the electrogenesis belongs to a component characterized by time constant of 1 msec, which is independent of an inhibiting field, and the rate of the second component strongly depends on inhibiting field we suggest that Cl--independent component belongs to release of chloride bound at site I (near the Schiff base) and this is accompanied by conformational changes of the protein. The latter is indirectly confirmed by results of barometric changes of kinetic rates [3] demonstrating (in contrast to bacteriorhodopsin) reduction of the volume of the hR molecule during the hR photocycle. We may speculate that chloride-anion release is a “squeezing out”. Naturally, the external field insignificantly influences the rate of opsin conformation change.
We assign the external field-dependent component to chloride transfer from the surface site III to site I located near the Schiff base. The latter reaction occurs due to KD difference (0.5 and 5 mM) and its rate is significantly influenced by the value of an electrostatic barrier.
This work was supported by the Russian Foundation for Basic Research (grant No. 00-04-48588).
REFERENCES
1.Kalaidzidis, I. V., Kalaidzidis, Y. L., and Kaulen,
A. D. (1998) FEBS Lett., 427, 59-63.
2.Varo, G., Brown, L. S., Sasaki, J., Kandori, H.,
Maeda, A., Needleman, R., and Lanyi, J. K. (1995) Biochemistry,
34, 14490-14499.
3.Varo, G., Needleman, R., and Lanyi, J. K. (1995)
Biochemistry, 34, 14500-14507.
4.Chizhov, I., and Engelhard, M. (2001) Biophys.
J., 81, 1600-1612.
5.Ludmann, K., Ibron, G., Lanyi, J. K., and Varo, G.
(2000) Biophys. J., 78, 959-966.
6.Muneyuki, E., Shibazaki, Ch., Wada, Yo.,
Yakushizin, M., and Ohtani, H. (2002) Biophys. J., 83,
1749-1759.
7.Otomo, J. (1996) Biochemistry, 35,
6684-6689.
8.Schobert, J., Lanyi, J., and Oesterhelt, D. (1986)
J. Biol. Chem., 25, 2690-2696.
9.Okuno, D., Asaumi, M., and Muneyuki, E. (1999)
Biochemistry, 38, 5422-5429.
10.Kalaidzidis, Ya. L., Gavrilov, A. V., Zaitsev, P.
V., Kalaidzidis, A. L., and Korolev, E. V. (1997)
Programmirovanie, 4, 38-46.
11.Golub, G., and Kahan, W. (1965) SIAM J. Num.
Anal., 2, 205-224.
12.Henry, E. R., and Hofrichter, J. (1992) Meth.
Enzymol., 210, 129-192.
13.Balashov, S. P., Imasheva, E. S.,
Govindjee, R., and Ebrey, T. G. (1996) Biophys. J.,
70, 473-481.