Activation of Molecular Oxygen by Infrared Laser Radiation in Pigment-Free Aerobic Systems
A. A. Krasnovsky, Jr.1*, N. N. Drozdova1, A. V. Ivanov2, and R. V. Ambartsumian3
1Bach Institute of Biochemistry, Russian Academy of Sciences, Leninskii pr. 33, Moscow 119071, Russia; fax: (7-095) 954-2732; E-mail: phoal@mail.ru2Blokhin Russian Cancer Center, Russian Academy of Medical Sciences, Kashirskoe Shosse 24, Moscow 115478, Russia
3Lebedev Physics Institute, Russian Academy of Sciences, Leninskii pr. 53, Moscow 119991, Russia
* To whom correspondence should be addressed.
Received May 12, 2003; Revision received June 5, 2003
With the goal of mimicking the mechanisms of the biological effects of low energy laser irradiation, we have shown that infrared low intensity laser radiation causes oxygenation of the chemical traps of singlet oxygen dissolved in organic media and water saturated by air at normal atmospheric pressure. The photooxygenation rate was directly proportional to the oxygen concentration and strongly inhibited by the singlet oxygen quenchers. The maximum of the photooxygenation action spectrum coincided with the maximum of the oxygen absorption band at 1270 nm. The data provide unambiguous evidence that photooxygenation is determined by the reactive singlet 1Deltag state formed as a result of direct laser excitation of molecular oxygen. Hence, activation of oxygen caused by its direct photoexcitation may occur in natural systems.
KEY WORDS: singlet oxygen, oxygen photoactivation, infrared laser radiation
It is known that photodynamic oxygenation reactions occur due to intermediate involvement of active oxygen species, among which excited oxygen molecules in the singlet 1Deltag-state (1O2) play the most important role. Usually the 1O2 responsible for these reactions appears as a result of energy transfer to O2 from triplet molecules of pigment photosensitizers. However, biological effects are also induced by low-intensity red or infrared laser irradiation of systems where pigment photosensitizers are not detected or their concentrations are very low [1-3]. The maxima of the action spectra of certain biological effects resemble the absorption maxima of the dimers ((O2)2) and monomer (O2) of oxygen molecules; therefore, it was proposed that some of these effects are initiated by reactive 1O2 molecules produced as a result of laser excitation of molecular oxygen dissolved in biological structures [4-7]. A chemical basis for this concept was given in papers by Evans [8] and Matheson and Lee [9], who showed that red and infrared light caused oxygenation of chemical traps of singlet oxygen dissolved in 1,1,2-trichloro-1,2,2-trifluoroethane saturated with oxygen at 130 atm. Using a set of the interference light filters, Evans estimated the action spectrum of this reaction and found that the maxima of the action spectrum correlated with the absorption maxima of dimer and monomer oxygen molecules [8].
On the other hand, it is known that the extinction coefficients corresponding to the IR absorption maxima of molecular oxygen are very low, 7-8 orders less than that of the porphyrin Soret band, and therefore the probability of direct excitation of oxygen is also very low [10]. The experiments by Evans [8] and Matheson and Lee [9] were done using very high oxygen concentration, which was more than that in biological systems by 4-5 orders of magnitude. Moreover, in the solvent they used the 1O2 lifetime was 30-100 msec, that exceeds by 5 orders the 1O2 lifetime in biological systems [11]. Thus, the solutions studied in these experiments [8, 9] can not serve as models adequate to living cells. Therefore, a goal of the present work was to investigate the efficiency of direct photoexcitation of molecular oxygen under natural conditions using laser generators of approximately that power which was used in biological experiments.
MATERIALS AND METHODS
Two laser systems were employed in our experiments. One system, manufactured at the Russian Cancer Center, consisted of an array of continuous GaAlAs diode lasers, radiation of which was focused into a fiber light guide. The wavelength and power of radiation eliciting the fiber light guide were 1266 ± 4 nm and 55 mW, respectively. After the light guide, the laser radiation passed through an infrared cut-off light filter transmitting light at lambda >= 900 nm. The transmission of the filter at 1266 nm was 90%. Hence, the radiation power at the surface of the irradiated sample was 50 mW. The second system, assembled at the Institute of Physics of the Russian Academy of Sciences, consisted of a wavelength-tunable forsterite laser pumped by a Nd-YAG laser with acoustooptical Q switch operated with 25 kHz repetition rate. It allowed us to obtain monochromatic infrared radiation at 1200-1290 nm with 5 nm bandwidth and 30-150 mW power.
The well known traps of singlet oxygen tetracene and 1,3-diphenylisobenzofuran (DPIBF) (Aldrich, USA) were used as substrates of oxygenation [12-16]. The interaction of these compounds with 1O2 is known to be purely chemical and is accompanied by formation of endoperoxides, having no absorption maxima in the visible spectral region. The rate constants of 1O2 reactions with DPIBF and tetracene are ~109 and ~107 M-1*sec-1 [12-16]. The molar absorption coefficients corresponding to the main absorption maxima of these traps are 2.35*104 and 1.25*104 M-1*cm-1, respectively [12, 13, 17]. Use was made of solutions whose absorbance at the principal absorption maxima of the traps varied from 0.3 to 1.5 in a 10-mm cell, which corresponded to the concentrations of the traps equal to not less than 25 µM. The solvents were carbon tetrachloride (“chemically pure”, Avogadro, Moscow), ethanol (96%, highest purification, Crystal, Orel), and heavy water (deuterium oxide) (Izotop, St. Petersburg). Solutions of the traps were illuminated in a 10-mm rectangular quartz cell. The volume of the solutions was 1.5 ml. The laser beam was focused so that the diameter of the illuminated spot on the surface of the cell was 9 mm. The absorption spectra of the solutions were measured using a Hitachi-3400 spectrophotometer (Japan). The experiments were performed as follows. At first, the absorption spectra of the air-saturated solutions of tetracene or DPIBF were measured before IR-irradiation and the absorbances in the main absorption maxima were recorded (A0). Then the solutions were irradiated for 15-60 min, shaken, and the absorbances were measured again (Af). The differences DeltaA = A0 - Af were used as the measures of the 1O2 formation rates.
RESULTS AND DISCUSSION
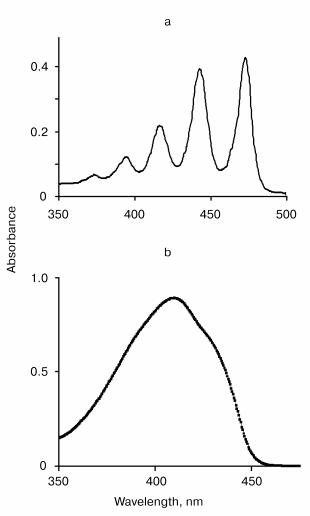
The absorption spectra of tetracene and DPIBF solutions are shown in the figure. In NNl4, the main maxima of the absorption spectra were located at 474 and 415 nm, respectively. Illumination of air-saturated solutions in this solvent by the radiation of the diode laser caused an appreciable bleaching of the absorption bands. The photodestruction rate (vr, M/sec) was calculated from measuring the illumination time needed to decrease the absorbance so that DeltaA = 0.1. This caused 8 and ~4 µM decreases of the tetracene and DPIBF concentrations, respectively. In the tetracene solutions, this was reached after ~20 min; in the DPIBF solutions, after ~10 min irradiation. The rate of photodestruction did not depend on the concentrations of the traps within the above-indicated range. After 15 min of purging with oxygen a fivefold increase in the photodestruction rates was observed; this was directly proportional to the increase in the oxygen concentration in the solutions. The 1O2 quencher beta-carotene (10 µM) strongly inhibited the photoreaction. In oxygen-free solutions obtained after 15 min purging with argon the photodestruction of the traps was not observed. The data clearly show that the photooxygenation of the traps is a result of their reaction with singlet oxygen that is formed due to laser excitation of oxygen molecules.
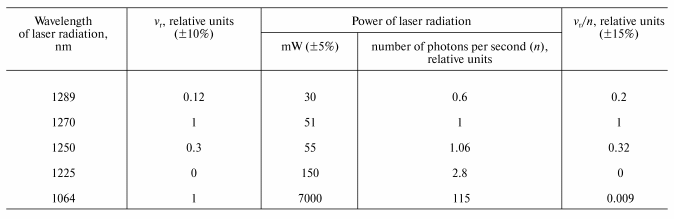
Final evidence supporting this mechanism was obtained from the measurement of the action spectra using the wavelength-tunable forsterite laser. The table illustrates the results of this experiment. The column vr/n in the table indicates the photoreaction action spectrum, where vr is the rate of tetracene photodestruction and n is a number of photons of IR radiation. Both parameters are indicated in relative units compared to their values, corresponding to the laser irradiation at 1270 nm. The table shows that the maximum (1270 nm) and the half bandwidth of the action spectrum correspond (within the range of the experimental errors) to those of the absorption spectrum of the electronic transition 3Sigmag- --> 1Deltag in molecular oxygen [10]. It was also found that 1O2 is formed upon excitation of the vibrational oxygen absorption band by the Nd-YAG laser band at 1064 nm. The effectiveness of this excitation is ~100 times less that of 1270 nm, which correlates with the Frank-Condon factor for this vibrational transition.The absorption spectra of tetracene in CCl4 (a) and 1,3-diphenylisobenzofuran in ethanol (b)
Similar measurements were performed in air-saturated solutions of DPIBF in ethanol, deuterium oxide, and water. Tetracene is not soluble in these media.
Rate of tetracene photodestruction (vr, M/sec) in
CCl4 under the action of laser radiation of different
wavelengths
In ethanol, IR radiation of the diode laser also caused bleaching of the main absorption band, whose maximum in this solvent was at 411 nm. During 60 min IR irradiation of the solution having 1.0 absorbance at 411 nm (~40 µM DPIBF) caused absorbance reduction DeltaA = 0.105 ± 0.005. This corresponds to the ~4 µM reduction of the DPIBF concentration. Hence, the rate of DPIBF photodestruction in ethanol was approximately three times less than in NNl4. After 15 min purging with oxygen, the photodestruction rate increased by a factor of 5. Addition of 1 mM sodium azide caused fourfold reduction in the photodestruction rate. The action spectrum of DPIBF photodestruction estimated using the forsterite wavelength-tunable laser resembled that obtained with tetracene in NNl4 (table). The maximum was also observed at approximately 1270 nm.
Measurements in heavy water were complicated by the low solubility of DPIBF in this medium. The best results were obtained in solutions containing 0.2% emulgator Cremafore 6E (Sigma, USA). In this experiment the absorbance at the maximum of the DPIBF absorption spectrum (419 nm) was 1.0 in the 10 mm cell. After 60 min irradiation by the IR emission of the diode laser the absorbance decreased, DeltaA = 0.08 ± 0.02. Photodestruction increased after purging with oxygen.
In water, the photoreaction rate was much less than in deuterium oxide and ethanol because the 1O2 lifetime in water is much less than in these media (four times less than in ethanol), that strongly reduces the measurable reactivity of 1O2 [11]. In addition, water strongly absorbs light at 1270 nm. The absorbance of the 10-mm water layer at this wavelength is about 0.5. We could not observe reliable DPIBF photodestruction in the air-saturated water solution containing 0.2% Cremafore 6E and DPIBF (DeltaA0 = 1.0 at 419 nm in the 10 mm cell) after 60 min irradiation by the diode laser. However, after purging with oxygen, 60 min irradiation caused appreciable bleaching of DPIBF, DeltaA = 0.060 ± 0.02.
Thus, the data indicate that the 1270 nm IR radiation, the intensity of which is close to its intensity in sun light, causes excitation of oxygen molecules dissolved in organic media and water saturated with air at normal atmospheric pressure that leads to population of the 1Deltag state followed by readily observed oxygenation of organic compounds:
3O2 + hnu (1270 nm) --> 1O2 (1Deltag),
1O2 (1Deltag) + organic substrates --> oxygenation.
Hence, similar reactions are possible in living cells, which correlates with published data [4-7]. However, the concentration of free oxygen in the structures of living cells is known to be 10-50 times less than in air-saturated solvents. The low oxygen concentration is a result of the activities of the respiration systems, which are the source of energy and also a natural biochemical mechanism decreasing formation of the active oxygen species [18]. This process certainly decreases the probability of photoexcitation of free oxygen and its involvement into metabolism of the living cells, though it cannot entirely exclude such reaction. On the other hand, one can assume that photoexcitation of oxygen molecules located inside key structures responsible for energy or regulatory processes, for instance, oxygen bound to oxidases or hemoglobin, is much more biologically efficient. It is noteworthy in this connection that the concentration of bound oxygen is much higher than that of free oxygen in living organisms.
This work was supported by the Russian Foundation for Basic Research (grant No. 01-03-32821a) and the International Science and Technology Center (grant No. 1552).
REFERENCES
1.Gamaleya, N. F., Shishko, Å. D., and Yanish,
G. B. (1983) Dokl. AN SSSR (Biofizika), 273, 227-231.
2.Karu, T. (2001) Uspekhi Sovrem. Biol.,
121, 110-120.
3.Klebanov, G. I., and Poltanov, E. A. (2003)
Laser Physics, 13, 1-14.
4.Ambartsumian, R. V. (1987) Proc. SPIE,
701, 341-343.
5.Ambartsumian, R. V., Eliseev, P. G., Eremeev, B.
V., Zakharov, S. D., et al. (1987) Lebedev Institute Report,
10, 35-38.
6.Danilov, V. P., Zhakharov, S. D., Ivanov, A. V., et
al. (1990) Dokl. AN SSSR (Biofizika), 311, 1255-1258.
7.Zakharov, S. D., and Ivanov, A. V. (1999)
Quantum Electronics, 29, No. 12, 1031-1053.
8.Evans, D. F. (1969) Chem. Comm.,
367-368.
9.Matheson, I. B. C., and Lee, J. (1970) Chem.
Phys. Lett., 7, 475-476.
10.Long, C., and Kearns, D. R. (1973) J. Chem.
Phys., 59, 5729-5736.
11.Krasnovsky, A. A., Jr. (1998) Membr. Cell.
Biol., 12, No. 5, 665-690.
12.Young, R. H., Brewer, D., and Keller, R. A.
(1973) J. Amer. Chem. Soc., 95, 375-379.
13.Merkel, P. B., and Kearns, D. R. (1975) J.
Amer. Chem. Soc., 97, 462-463.
14.Stevens, B., Perez, S. R., and Ors, J. A. (1974)
J. Amer. Chem. Soc., 96, 6846-6850.
15.Krasnovsky, A. A., Jr. (1979) Photochem.
Photobiol., 29, 29-36.
16.Krasnovsky, A. A., Jr., Venediktov, Å. A.,
and Chernenko, O. N. (1982) Biofizika, 27,
1009-1016.
17.Clare, E. (1971) Polycyclic Hydrocarbons
[Russian translation], Khimiya, Moscow, p. 375.
18.Skulachev, V. P. (1995) Mol. Biol.
(Moscow), 29, 1199-1209.