DNA-Hydrolyzing Antibodies from Sera of Autoimmune-Prone MRL/MpJ-lpr Mice
V. V. Dubrovskaya1, A. S. Andryushkova2, I. A. Kuznetsova1, L. B. Toporkova3, V. N. Buneva1,2, I. A. Orlovskaya3, and G. A. Nevinsky1,2*
1Novosibirsk Institute of Bioorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, pr. Lavrent'eva 8, Novosibirsk 630090, Russia; fax: (3832) 333-677; E-mail: nevinsky@niboch.nsc.ru2Novosibirsk State University, ul. Pirogova 2, Novosibirsk 630090, Russia
3Institute of Clinical Immunology, Siberian Branch of the Russian Academy of Medical Sciences, ul. Yadrentsovskaya 14, Novosibirsk 630099, Russia
* To whom correspondence should be addressed.
Received May 13, 2003
Catalytically active antibodies (abzymes) hydrolyzing proteins, polysaccharides, ATP, DNA, and RNA have been detected in the sera of patients with various autoimmune and some viral diseases, but abzymes from the sera of animals are practically unstudied. The development of lupus-like autoimmune disease of MRL/MpJ-lpr mice is an experimental model for study of autoimmune pathologies and immunopathogenesis. In this work, homogeneous IgG preparations were isolated from the sera of MRL/MpJ-lpr mice. These antibodies (Abs), their Fab-fragments, and isolated light chains were shown to possess catalytic activity in DNA hydrolysis, whereas Abs from the sera of control healthy mice did not hydrolyze DNA. The data demonstrate that DNA hydrolyzing activity is an intrinsic property of Abs from MRL/MpJ-lpr mice. It was shown that various markers of autoimmune pathologies (level of total protein concentration in urea (proteinuria), Abs titers to native and denatured DNA, and DNA-hydrolyzing activity of IgG) increased in animals with aging, but they noticeably increased (2-22 times) only after appearance of obvious indicators of pathology independently of age. The highest increase in proteinuria (25-fold), anti-DNA Abs titers (12-19-fold), and abzyme activity (120-fold) was found in mice after their immunization with DNA-protein complex.
KEY WORDS: MRL/MpJ-lpr mice sera, immunoglobulins, native abzymes, DNA hydrolysis
Abbreviations: Ab) antibody; AD) autoimmune diseases; SLE) systemic lupus erythematosus; DTT) dithiothreitol; OA) optical absorption.
Antibodies (Abs) raised against stable analogs of the transition state
complexes of catalytic reactions capable of catalyzing more than 100
various reactions have been described [1-8]. Natural abzymes with proteolytic, nuclease,
phosphatase, and amylolytic activities have been detected in the blood
of patients with various autoimmune (bronchial asthma, systemic lupus
erythematosus, autoimmune thyroiditis, polymyositis, polyarthritis, and
multiple sclerosis) and some viral (hepatitis, AIDS) diseases (for
review, see [6, 9-11]). However, abzymes have not been found in the
blood of healthy donors and patients with influenza, pneumonia,
tuberculosis, tonsillitis, duodenal ulcer, and some types of cancer
proceeding without immune status disorder [9-11].
DNA and anti-DNA Abs at increased concentrations, which are considered to arise from cell apoptosis, can be detected in the blood of patients with some autoimmune diseases (AD) [9-11]. Apoptotic cell antigens are often recognized as auto-Abs targets [9-11]. Many anti-DNA Abs are directed against histone-DNA nucleosomal complexes appearing as a result of internucleosomal cleavage during apoptosis. Apoptotic cells are the primary source of antigen and immunogen in SLE, and these features in recognition, perception, processing, and/or presentation of apoptotic auto-antigen by antigen-presenting cells can cause autoimmune processes.
In AD, spontaneous accumulation of primary Abs against proteins, nucleic acids, and their complexes occurs, and then secondary Abs forms to already accumulated primary Abs, and so on [12, 13]. Thus, in the blood of patients with AD various Abs can be found; there can be Abs directly to antigen with changed conformation (transition state analog) or antiidiotypic Abs whose appearance can be explained using Jerne's antiidiotypic net model [14]. According to the accepted concept, the presence of abzymes in blood indicates development of autoimmune processes in the human body [9-11].
The presence of natural abzymes in donors without any immune status pathology was considered impossible for a long time. However, we demonstrated that autoimmune processes similar to that in AD patients occur in apparently healthy women during pregnancy and immediately after childbirth [15]. Because of the specific state of the immune system of pregnant and especially lactating women, they are subject to immunization by external (nutritional) as well as internal immunogens [16-18]. The blood of such women, like that of AD patients, contains DNA at increased concentrations [19-21] and fetal cells at low concentrations [22]. We were first to describe human breast milk abzymes sIgA in the breast milk of healthy women in childbirth that catalyze protein phosphorylation [23-25]. Later it was shown that small subfractions of breast milk IgG and sIgA catalyze hydrolysis of DNA, RNA [16-18, 26], ribo- and deoxyribo-NMP, -NDP, and -NTP [27], polysaccharides [28], and also cleavage of DNA and RNA 5´-terminal phosphate (phosphatase activity) [17, 18]. It is interesting that human breast milk Abs possess higher activity than most abzymes of AD patients [9-11].
These data raised several completely new questions about the origin and mechanisms of abzyme accumulation in mammals. However, these questions can be answered only using experimental animals for modeling certain states of the immune system. Mice provide the most convenient models; however, by they have not yet been widely used for study of the mechanisms of abzyme accumulation. DNA- and RNA-hydrolyzing activities of monoclonal antibodies obtained by immunization of mice with various DNA are described in [29, 30].
Spontaneous SLE-like disease of MRL/MpJ-lpr mice is an experimental model for study of the pathogenesis of autoimmune pathologies. The lpr gene bears a mutation causing a defect in the Fas molecule responsible for cell apoptosis [31]. Decrease in apoptotic function results in accumulation of a large number of so-called binegative T-cells (CD4-CD8-, B220+, TCR+) in peripheral lymphoid organs. Definite lymphoadenopathy is accompanied by production of various auto-antibodies, immune complexes, and glomerulonephritis [32]. Along with cell apoptosis disorder, many other factors contribute to genesis of animal autoimmune pathology. Disorder in proliferation and differentiation of hemopoietic stem cells is now considered as one of the most important AD origins. Some authors consider systemic and organ-specific AD as polyclonal abnormal stem cell proliferative syndrome [33]. Earlier it was shown that stem cells of MRLlpr/lpr mice are noticeably more radio-resistant than stem cells of normal mice; increase in the number of bone-marrow hemopoietic precursors of various cells with aging is also noted [34].
We recently studied colony-forming capacity of bone-marrow hemopoietic precursors during the development of AD in MRL/MpJ-lpr mice [35, 36]. A normal ratio of hemopoietic precursors was detected in bone marrow of young MRL/MpJ-lpr mice, as described for normal non-autoimmune mice. Changes in the level of proliferation and direction of hemopoietic stem cell differentiation were observed in mice with definitely manifested disease. The data indicate that MRL/MpJ-lpr mice can be a suitable model for study of abzyme accumulation mechanisms and their correlation with disorder in stem cell proliferation and differentiation.
The goal of this work was to demonstrate DNA-hydrolyzing activity of antibodies from the blood of MRL/MpJ-lpr mice using the methods described in the literature. The relative activities of such Abs in hydrolysis of DNA from blood of young healthy subjects, after spontaneous appearance of AD symptoms, and as a result of the immune response stimulation by immunization of animals with DNA-protein complex were compared.
MATERIALS AND METHODS
Serum preparations. MRL/MpJ-lpr mice and Balb/c and (CBA×C57BL)F1 control mice were kept under standard conditions; the animals were from 1.5- to 7-month-old. To obtain Abs, we used sera prepared according to the standard procedure by addition of 4% sodium citrate to blood samples (1/4 of blood volume) and subsequent removal of cells by centrifugation (2000 rpm, 10 min).
Immunization of mice. Mice were immunized with 20-40 µg antigen DNA per mouse. A conjugate of high-molecular-weight thymus DNA with methylated BSA dissolved in physiological solution was used as antigen. A mixture of 0.5 volume of complete Freund's adjuvant and 0.5 volume of antigen solution was used. The mixture was stirred to form a homogeneous gel and injected subcutaneously or into paw pads. Two further immunizations with a mixture containing incomplete Freund's adjuvant were performed after 14 and 23 days, respectively. Immunization results were analyzed a month after the first immunization.
Estimation of the level of proteinuria. Calibration BSA solutions (1-2 µl) at concentrations from 0.1 to 4 mg/ml and then 1-2 µl of mouse urine were applied on Whatman 3 MM paper. The paper was dried (5 min), wet with acetone for protein fixation, and dried again for 5 min. Then the paper was put into solution for protein staining (45% methanol, 10% acetic acid, 0.25% Coomassie Blue R250) for 5 min, and excess dye was washed out with solution containing 10-20% butanol and 5% acetic acid to disappearance of background staining. Then the paper was rinsed with water and dried. Protein concentration in mouse urine was determined by scanning of spot density using a calibration curve obtained for BSA.
Enzyme immunoassay of anti-DNA antibody concentration. We adapted and optimized a procedure for enzyme immunoassay of titers of anti-DNA Abs using standard test-system plates with immobilized double- and single-stranded DNA from Specialized Scientific Laboratories (Moscow); this procedure was developed for analysis of titers of anti-DNA Abs in human blood. For blocking, 200 µl of TBS (25 mM Tris-HCl, pH 7.5, 150 mM NaCl) containing 0.01% sodium azide and 0.5% ovalbumin were added in each well and incubated for 1 h at 22°C. Then wells were washed with TBS containing 0.05% Triton X-100 (TBS-1, 3 times with 200 µl per well). Blood serum was 200 times diluted with blocking solution, and 50-µl samples of diluted serum were placed into each well and incubated for 1 h at 22°C. Then wells were washed with TBS-1 buffer (3 times with 200 µl per well) and treated with conjugate of Abs (50 µl, 2 µg/ml) against mouse IgG with peroxidase, and the plate was incubated for 1 h at 22°C. Then wells were washed with TBS-1 (2 times with 200 µl per well) and the last time with TBS. Liquid was carefully removed from wells, and 50 µl of solution containing tetramethyl benzidine and hydrogen peroxide in citrate-phosphate buffer (concentrations corresponded to those described for the standard test-systems) was placed into each well. After incubation for 10 min, the reaction was stopped by addition of 50 µl of 50% sulfuric acid. The relative anti-DNA Ab concentration in samples was expressed in optical absorption (OA) units at 445 nm of solution in wells (A445 units, the average of three measurements). OA was measured using a multichannel spectrophotometer (Pushchino, Moscow Region). For calculating mouse blood serum OA, absorption of control Abs not interacting with DNA was taken into account.
Isolation of antibodies from mouse blood serum. Abs from mouse blood was purified by a modification of a procedure earlier developed for isolation of abzymes from blood of patients with AD [37-39]. To precipitate proteins, ammonium sulfate was added to blood serum to 50% saturation, protein was isolated by centrifugation (12,000 rpm, 10 min), and the pellet was dissolved in the initial volume of TBS. This solution was applied on a column with protein A-Sepharose equilibrated with TBS. Proteins not interacting with the sorbent were washed off with TBS. Nonspecifically adsorbed proteins and lipids were eluted with TBS containing 0.5% Triton X-100. The IgG fraction was eluted with 40 mM glycine-HCl, pH 2.6, and immediately neutralized with 1.5 M Tris-HCl, pH 8.8. The obtained Abs fractions were dialyzed against several volumes of TBS and used for estimation of enzymatic activity.
IgG was separated from IgA and IgM by high-pressure gel filtration on a Superdex 200 HR 10/30 column (100 × 300 mm) (using a Sprint Biocad chromatograph from Pharmacia, Sweden) equilibrated with 20 mM Tris-HCl, pH 7.5, analogously to [15]. Before application on the column, the sample (0.2-0.3 ml) was incubated with TBS containing 2.5 M MgCl2 for 20 min at 20°C. Buffer containing 1.5 M MgCl2 and 1 M NaCl (2 ml) was applied on the column as a “salt pillow” before the sample. Abs were eluted with 20 mM Tris-HCl, pH 7.5 (0.2 ml/min).
It should be noted that abzymes purified on protein A-Sepharose are subject to so-called acidic shock; not every enzyme can sustain this shock, many of them completely losing their catalytic activity [9-11]. Abs slowly regain their activity after acidic shock on storage in neutral buffer for 0.3-2 months at 4°C. That is why Abs preparations purified as described above were used for analysis of their activity 2-5 and more weeks after the last purification stage. During this time some of them were partly destroyed, forming free light and heavy chains.
Chromatography of Abs on DNA-cellulose according to [25, 26] was the last stage of the standard purification procedure; at this stage, partly destroyed IgG molecules were separated from the native ones.
Acidic shock. High-pressure gel filtration of Abs under severe conditions was performed analogously to that for Abs isolation (see above). A column was equilibrated by acidic (50 mM glycine-HCl, pH 2.6, containing 0.3 M NaCl) or alkaline (50 mM potassium-phosphate, pH 10.5) buffer. Before gel filtration Abs were preincubated in the mentioned acidic buffer or 0.1 M KOH for 30 min at 30°C, as described in [40, 41].
Abs activity in DNA and RNA hydrolysis significantly decreased immediately after Abs treatment with acidic buffers. Activity was restored noticeably after dialysis against 500 volumes of buffer A for 10 h and subsequent incubation of solutions for a week or more at 4°C.
Abs activity in DNA hydrolysis was estimated as described earlier for abzymes from blood of AD patients [37-42]. The reaction mixture (20 µl) contained 20 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA, 10-20 µg/ml Bluescript plasmid DNA, and 0.05-0.2 mg/ml Abs. After incubation for 2-4 h at 37°C, 5 µl of solution containing 0.05% Bromophenol Blue, 50% glycerol, 20 mM Tris-HCl, pH 7.5, was added to the reaction mixture. The reaction products were analyzed by electrophoresis in 1% agarose gel; buffer for electrophoresis contained 40 mM Tris-acetate, pH 7.5, and 1 mM EDTA. After electrophoresis, DNA was stained with 0.5 µg/ml ethidium bromide. To estimate the relative activity, the gel was photographed and scanned.
Testing of DNA-hydrolyzing activity of antibodies in the gel. DNA-hydrolyzing activity of Abs in gel was estimated by SDS-PAGE (5-20% gradient polyacrylamide gel containing 5-20 µg/ml polymeric DNA from calf thymus analogously to [17, 18, 26, 39, 41]). After separation of proteins in the gel, the latter was washed free of SDS with 4 M urea for 1 h and then with water (10 portions of water for 5-7 min each). To restore Abs activity, the gel was incubated in 20 mM Tris-HCl, pH 7.5, containing 5 mM MgCl2 and 1 mM EDTA for 16 h at 37°C, and then stained with ethidium bromide. The gel portion where DNA was cleaved, was revealed as a dark spot on uniformly fluorescing background. Positions of protein bands were determined by staining of the gel with Coomassie Blue R-250.
Reagents. The following reagents were used in this study: acrylamide, N,N´-methylene-bis-acrylamide, sodium dodecyl sulfate (SDS), glycine, EDTA, and Triton X-100 from Merck (Germany); TEMED from Reanal (Hungary); MgCl2, Freund's adjuvant, sodium azide, and ethidium bromide from Sigma (USA); sodium citrate and agarose from Serva (Germany); Tris from INC (USA); sodium chloride from USB (USA); BSA from Euro Bio. Conjugate of Ab with horseradish peroxidase was kindly given by Dr. L. Matveev (Institute of Bioorganic Chemistry, Novosibirsk). Other reagents were of extra pure grade and of Russian production.
RESULTS AND DISCUSSION
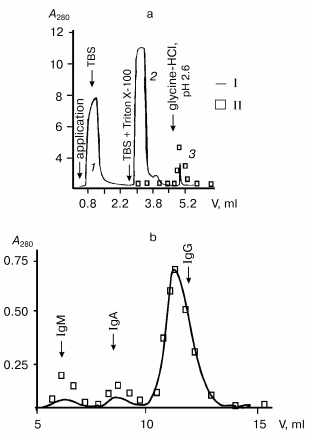
DNase activity of Abs from MRL/MpJ-lpr mouse sera was analyzed. A high level of proteinuria (protein concentration in urea >= 3 mg/ml) is a marker of autoimmune pathology in animals [43]. In the first stage of the work, mice with spontaneous autoimmune process and clearly manifested proteinuria (>8 mg/ml) were used. Isolation of Abs from mouse sera by affinity chromatography on protein A-Sepharose is presented in Fig. 1 as an example. After Abs adsorption, a column was washed with a buffer containing Triton X-100, which destroys even tightly bound noncovalent protein complexes. Subsequent washing of the column with more acidic buffer (pH 2.6) resulted in elution of peak 3; according to the electrophoretic data, this peak is a mixture of highly purified Abs (IgG, IgM, and IgA) without admixtures of non-immunoglobulin nature (data not illustrated here) [27, 44].
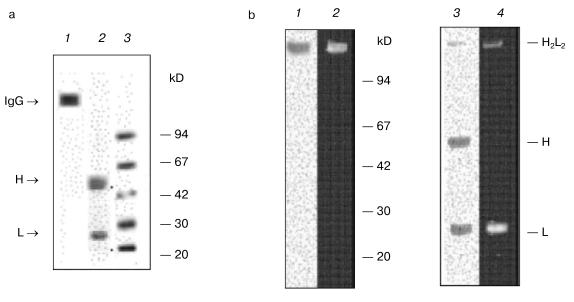
Subsequent separation of Abs by gel filtration on Superdex 200 HR under severe conditions (in the presence of MgCl2 and NaCl) yields catalytically active (Fig. 1) and electrophoretically homogeneous preparations of IgG, IgA, and IgM antibodies [27, 44]. Electrophoretic analysis of IgG preparations from mouse blood is presented in Fig. 2 as an example. As shown, abzymes are homogeneous before (Fig. 2a, lane 1) and after (Fig. 2a, lane 2) treatment with dithiothreitol (DTT). IgG preparations contained only heavy and light chains (H ~ 50 and L ~ 25 kD).Fig. 1. Profiles of optical absorption (I) and DNase activity (II) on affinity chromatography of protein from mouse sera on protein A-Sepharose (a) and subsequent high-pressure gel filtration of Abs (3) on Superdex 200 HR 10/30 (b).
In subsequent experiments only IgG preparations were used. For several experiments, Abs with increased affinity to DNA were additionally purified by affinity chromatography on DNA-cellulose.Fig. 2. a) Electrophoretic analysis of homogeneity of IgG preparations (silver-stained) from mouse blood before (1) and after treatment with DTT (2); 3) protein markers with known molecular masses. b) Analysis of IgG activity in situ in the DNA-containing gel (negatives are presented) (2, 4); protein staining with Coomassie Blue R250 after electrophoresis (1, 3): before (1) and after (2) partial reduction of disulfide bonds with DTT.
Evidence for nuclease activity of Abs. Catalytic activity of Abs is usually proved by checking 13 sufficiently rigid criteria (for review, see [9-11]). We checked some of these criteria and showed that (some data are not illustrated):
- Abs preparations were electrophoretically homogeneous (Fig. 2a, staining with silver);
- optical absorption profiles of proteins on gel filtration in acidic or alkaline buffer coincided with the profiles of DNA-hydrolyzing activity (IgG purified on DNA-cellulose were used);
- incubation of Abs with anti-IgG-Sepharose resulted in complete removal of DNA-hydrolyzing activity from solution;
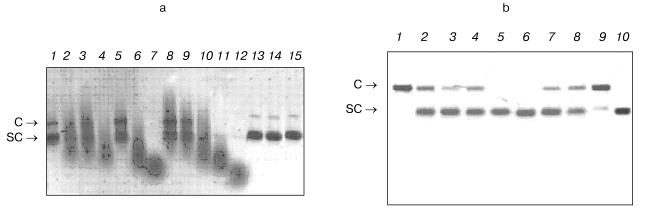
- homogeneous preparations of Abs Fab-fragments possessed DNA-hydrolyzing activity (Fig. 3b, lane 9, Fab corresponds to Ab of lane 1);
- DNase activity was detected only in Abs from urine of mice with definite proteinuria (usually mice 5-8-month-old), whereas Abs from sera of young mice (1-2-month-old) without definite markers of autoimmune reactions (Fig. 3a, lane 13) and also from sera of not autoimmune-prone Balb/c and (CBA×C57BL)F1 control mice (3-7-month-old) did not possess activity (Fig. 3a, lanes 13 and 14).
It should be noted that among the known criteria there are some undoubtedly indicating that directly Abs and not admixtures possess the activity. Detection of Abs activity in situ after protein separation by SDS-PAGE in the substrate-containing gel is the first of such criteria [9-11]. As we demonstrated earlier, when this most rigid criterion is met, other less rigid criteria are also met [9-11].Fig. 3. a) Analysis of DNase activity of IgG preparations from several mice of various ages and at various stages of autoimmune process (plasmid Bluescript DNA, negative). Lanes: 1-12) twelve MRL/MpJ-lpr mice; 13, 14) DNA incubation with Abs from control Balb/c and (CBA×C57BL)F1 mice, respectively; 15) DNA incubated in the absence of Abs. Plasmid forms: C) coiled; SC) supercoiled. Abs were added at concentration 0.1 mg/ml. b) Optimization of Abs concentration for quantitative analysis of DNase activity of IgG preparations for 8 diseased MRL/MpJ-lpr mice (lanes 1-8). Lanes: 9) Fab-fragment of IgG of one Abs preparation (lane 1); 10) DNA incubated in the absence of Abs. In each case Ab concentration was fitted so that only nicked coiled DNA (C) is formed from supercoiled DNA (SC). Abs concentration was thus varied from 0.1 to 100 µg/ml depending on preparation. Lane 1 corresponds to Abs of a mouse (1 µg per 20 µl of a mixture or 50 µg/ml) which activity was taken as an activity unit in calculations of the relative activity of other Abs preparations (table).
As shown in Fig. 2b, after SDS-PAGE of IgG (Abs purified on DNA-cellulose) in DNA-containing gel, hydrolysis of DNA before reduction of disulfide bonds is observed only in the gel portions corresponding to the positions of protein bands of the initial IgG (lane 2) and after reduction of disulfide bonds with DTT - only at the positions of Ab light chains (lane 4). This result undoubtedly indicates that catalytic activity is an intrinsic property of light chains of IgG from mouse blood, since in each of these cases other DNA cleavage sites are absent.
Thus, we first demonstrated that sera of autoimmune MRL/MpJ-lpr mice could contain DNA-hydrolyzing abzymes. Analogously to human IgG, DNase activity of Ig from mouse sera is caused by activity of Ab light chain. This is in accord with literature data that for most abzymes; catalytically active sites are positioned in the variable part of Ig light chains [9-11]. However, analysis of some natural abzymes and Abs induced by transition state analogs demonstrated that heavy as well as light Ab chains can participate in functioning of the active sites and sometimes the contribution of heavy chains in larger [9-11]. For example, both light and heavy chains participate in formation of DNA-hydrolyzing sIgA and ATP-hydrolyzing IgG from human breast milk [17, 27]. Active sites of the natural abzymes seem to be variously organized, but for DNA-hydrolyzing IgG from mouse sera, the active site is localized on the light chain, as for most Abs from sera of AD patients.
Dynamics of change in DNase activity of mouse sera Abs during aging and after immunization. As mentioned above, autoimmune pathologies of MRL/MpJ-lpr mice arise spontaneously. Usually, the appearance of visual symptoms is a definite marker of autoimmune pathology: pink spots, baldness of head and some areas of the back, general state worsening and so on; they are often observed at the age of 3-7 months. Definite proteinuria is observed together with other symptoms. It is known that auto-Abs formed in the mice react with DNA, histones, leucocytes, thrombocytes, and erythrocytes [45], and immune complexes thus formed cause complement activation, which results in development of glomerulonephritis. As a result, protein content in urea rises (proteinuria). It is assumed that proteinuria can be a marker of profound development of autoimmune reactions in mammals in AD, when kidney functions are significantly disrupted.
Comparison of the relative catalytic activity of Abs from mouse sera at various life periods was of special interest, since by now it is not known at what AD stage appearance of catalytically active Abs can be detected. We studied activity of Abs from sera of mice of various age: 1.5, 2.5, and 7 months (four animals of each age). Analysis of DNase activity of IgG in hydrolysis of supercoiled DNA by Ab preparations from several mice (IgG at equal concentrations were added) is presented in Fig. 3a as an example. However, the relative activity of Abs in hydrolysis of DNA from blood markedly differed from mouse to mouse. Figure 3a presents a part of the cleavage spectrum of plasmid DNA by antibodies from several mice during 2 h. As can be seen, some Abs cause only single breaks in supercoiled DNA during this time (lanes 1, 5, 8, and 9) and others cause multiple breaks thus forming linear DNA (lanes 2, 3, and 10). Most active Abs hydrolyze DNA to short- and middle-length oligonucleotides (lanes 4, 6, 7, 11, and 12). Based on these data, quantitative estimation of the level of DNA hydrolysis appeared to be rather complicated.
For quantitative comparison of change in the relative IgG activity in the course of spontaneous appearance of mouse SLE symptoms, in each case such Ab concentration was fitted that during 2 h incubation supercoiled DNA is converted into the coiled nicked form without formation of linear DNA and low-molecular-weight products of its hydrolysis (Fig. 3b). Activity of Ab preparation of a mouse in which 1 µg of Abs converted completely (~100%) all supercoiled DNA into the coiled nicked form (Fig. 3b, lane 1) was taken as an activity unit. The relative activity of other Ab preparations was estimated accounting the percent of SC DNA conversions into the coiled nicked form and Abs concentration used. Abs activity was analogously analyzed for DNA-immunized mice. The results are presented in the table along with the data on Abs titers against the native and denatured DNA in the sera and protein concentration in mouse urine.
Characteristics of autoimmune process in MRL/MpJ-lpr mice
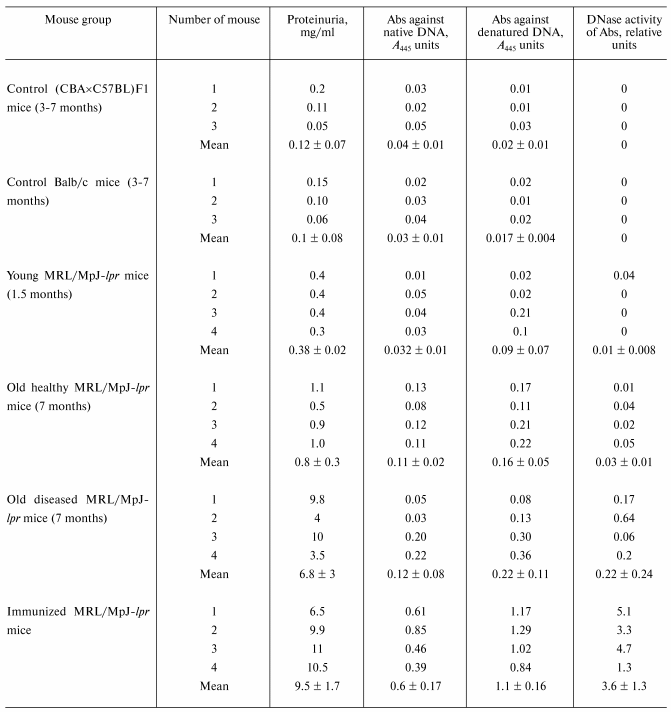
*Activity of antibody preparation of a mouse in which
1 µg of Abs converted almost completely (~100%) supercoiled
DNA into the linear form after 2 h incubation of the reaction
mixture was taken as an activity unit.
As shown in the table, IgG from sera of not autoimmune-prone control Balb/c and (CBA×C57BL)F1 mice do not possess DNase activity. For these mice, definite proteinuria is also not observed, and Abs titers against the native and denatured DNA are very low. For young MRL/MpJ-lpr mice (approximately equal data for 1.5- and 2.5-month-old mice) with anti-DNA Abs concentration and the level of proteinuria comparable with those for control mice, DNase activity of Abs in most cases is also absent or is very low (table).
It is interesting that some MRL/MpJ-lpr mice can exhibit no definite SLE symptoms for a rather long time, i.e., up to 7-8 months. The level of anti-DNA Abs and proteinuria of four such mice was analyzed. It was found that protein concentration in urea of these mice can be ~2 times higher than that of young mice (table). Anti-DNA Abs concentration in sera also increased ~2-3 times. The level of DNase activity of Abs increases ~3 times but still remains relatively low (table).
Spontaneous SLE of MRL/MpJ-lpr mice (7-month-old mice were used) first results in drastic increase (~18 times) in the average proteinuria level (table). However, anti-DNA Abs titer does not change so drastically (only ~2.4-3.4 times) compared with that for young mice. DNase activity of Abs increases ~22 times on average. In spontaneous SLE this can indicate preferential activation of immunocompetent cells producing abzymes and to a lesser extent, cells synthesizing anti-DNA Abs without catalytic activity. As a whole, this corresponds with our data that the titer of Abs subfraction with DNase activity in the blood of patients with SLE and multiple sclerosis increases in acute period more rapidly than the total anti-DNA Abs titer [11].
Immunization of animals with a DNA complex with methylated BSA has maximal effect on the level of anti-DNA Abs and their catalytic activity (table). Increase in the level of proteinuria (25 times) is comparable with that for mice with spontaneous SLE (18 times), but increase in the total anti-DNA Abs titer (12-18 times) and DNase activity of the abzyme fraction (120 times) is ~5 times higher in case of immunization.
So, catalytically active Abs with DNase activity can be synthesized as a result of spontaneous SLE of MRL/MpJ-lpr mice. However, the relative level of abzyme activity as well as titers of anti-DNA antibodies remain relatively low and increase drastically on immunization of mice with DNA-protein complex.
This work was financially supported by the Russian Foundation for Basic Research (grant No. 02-04-49350) and the 6th Expert Competition of Scientific Projects of Young Scientists of the Russian Academy of Sciences in Basic and Applied Research for 1999 (grant No. 234).
REFERENCES
1.Benkovic, S. J. (1992) Annu. Rev. Biochem.,
61, 29-54.
2.Hilvert, D. (1992) Pure Appl. Chem.,
64, 1103-1113.
3.Stewart, J. D., and Benkovic, S. J. (1993) Chem.
Soc. Rev., 22, 213-219.
4.Suzuki, H. (1994) J. Biochem., 115,
623-628.
5.Schultz, P. G., and Lerner, R. A. (1995)
Science, 269, 1835-1842.
6.Nevinsky, G. A., Semenov, D. V., and Buneva, V. N.
(2000) Biochemistry (Moscow), 65, 1233-1244.
7.Generalov, I. I., and Novikov, D. K. (1998)
Uspekhi Sovrem. Biol., 118, 178-193.
8.Nevinsky, G. A., Kanyshkova, T. G., Semenov, D. V.,
and Buneva, V. N. (2001) Vestnik RAMN, 2, 38-45.
9.Nevinsky, G. A., Kanyshkova, T. G., and Buneva, V.
N. (2000) Biochemistry (Moscow), 65, 1245-1255.
10.Nevinsky, G. A., Favorova, O. O., and Buneva, V.
N. (2002) in Protein-Protein Interactions; A Molecular Cloning
Manual (Golemis, E., ed.) Cold Spring Harbor, pp. 523-534.
11.Nevinsky, G. A., and Buneva, V. N. (2002)
Meth. Immunol., 269, 235-249.
12.Raptis, L., and Menard, H. A. (1980) J. Clin.
Invest., 66, 1391-1399.
13.Reimer, C., Raska, I., Tan, E. M., and Sheer, U.
(1987) Virchows Arch., 54, 131-136.
14.Jerne, N. K. (1974) Ann. Immunol.,
125, 373-398.
15.Buneva, V. N., Kudryavtseva, A. N., Gal'vita, A.
V., Dubrovskaya, V. V., Khokhlova, O. V., Kalinina, I. A., Galenok, V.
A., and Nevinsky, G. A. (2003) Biochemistry (Moscow), 68,
890-900.
16.Kanyshkova, T. G., Semenov, D. V., Vlassov, A.
V., Khlimankov, D. Yu., Baranovsky, A. G., Shipitsyn, M. V., Yamkovoi,
V. I., Buneva, V. N., and Nevinsky, G. A. (1997) Mol. Biol.
(Moscow), 31, 1082-1091.
17.Nevinsky, G. A., Kanyshkova, T. G., Semenov, D.
V., Vlassov, A. V., Gal'vita, A. V., and Buneva, V. N. (2000)
Appl. Biochem. Biotechnol., 83, 115-129.
18.Buneva, V. N., Kanyshkova, T. G., Vlassov, A. V.,
Semenov, D. V., Khlimankov, D. Yu., Breusova, L. R., and Nevinsky, G.
A. (1998) Appl. Biochem. Biotechnol., 75, 63-76.
19.Mohan, C., Adams, S., Stanic, V., and Datta, S.
K. (1993) J. Exp. Med., 177, 1367-1381.
20.Teodoresku-Eskarku, I. (ed.) (1981) Human
Reproduction, Medical Publishers, Bucharest, pp. 703-725.
21.Kazakov, V. I., Bozhkov, V. M., Linde, V. A.,
Repina, M. A., and Mikhailov, V. M. (1995) Tsitologiya,
37, 232-235.
22.Amino, N., Tada, H., and Hitaka, Y. (1999)
Thyroid, 9, 705-713.
23.Kit, Yu. Ya., Semenov, D. V., and Nevinsky, G. A.
(1995) Mol. Biol. (Moscow), 29, 893-906.
24.Kit, Yu. Ya., Semenov, D. V., and Nevinsky, G. A.
(1996) Biochem. Mol. Biol. Int., 39, 521-527.
25.Nevinsky, G. A., Kit, Yu. Ya., Semenov, D. V.,
and Buneva, V. N. (1998) Appl. Biochem. Biotechnol., 75,
77-91.
26.Kanyshkova, T. G., Semenov, D. V., Khlimankov, D.
Yu., Buneva, V. N., and Nevinsky, G. A. (1997) FEBS Lett.,
416, 23-27.
27.Semenov, D. V., Kanyshkova, T. G., Kit, Yu. Ya.,
Khlimankov, D. Yu., Akimzhanov, A. M., Gorbunov, D. A., Buneva, V. N.,
and Nevinsky, G. A. (1998) Biochemistry (Moscow), 63,
935-943.
28.Savel'ev, A. N., Eneyskaya, E. V., Shabalin, K.
A., Filatov, M. V., and Neustroev, K. N. (1999) Protein Peptide
Lett., 6, 179-184.
29.Andrievskaya, O. A., Kanyshkova, T. G., Yamkovoi,
V. I., Buneva, V. N., and Nevinsky, G. A. (1997) Doklady RAN,
355, 401-404.
30.Gololobov, G. V., Rumbley, C. A., Rumbley, J. N.,
Schourov, D. V., Makarevich, O. I., Gabibov, A. G., Voss, E. W., Jr.,
and Rodkey, L. S. (1997) Mol. Immunol., 34, 1083.
31.Nagata, S., and Suda, T. (1995) Immunol.
Today, 16, 39-43.
32.Watanabe-Fukunada, R., Brannan, C. I., and
Copeland, N. G. (1992) Nature, 356, 314-317.
33.Ikehara, S., Kawamura, M., and Takao, F. (1990)
Proc. Natl. Acad. Sci. USA, 87, 8341-8344.
34.Ishida, T., Inaba, M., and Hisha, H. (1994) J.
Immunol., 152, 3119-3123.
35.Orlovskaya, I. A., Dubrovskaya, V. V., Toporkova,
L. V., Chernykh, E. R., Tikhonova, M. A., Sakhno, L. V., Buneva, V. N.,
Kozlov, V. A., and Nevinsky, G. A. (2002) Proc. Third Int. Conf. on
Bioinformatics of Genome Regulation and Structure, Novosibirsk,
July 14-20, 2002, Vol. 4, pp. 49-51.
36.Toporkova, L. B., Dubrovskaya, V. V., Sakhno, L.
V., Tikhonova, M. A., Chernykh, E. R., Buneva, V. N., Nevinsky, G. A.,
Kozlov, V. A., and Orlovskaya, I. A. (2002) Rus. J. Immunol.,
7, 245-250.
37.Baranovsky, A. G., Kanyshkova, T. G.,
Mogil'nitsky, A. S., Naumov, V. A., Buneva, V. N., Gusev, E. I., Bojko,
A. N., Zargarova, T. A., Favorova, O. O., and Nevinsky, G. A. (1998)
Biochemistry (Moscow), 63, 1239-1248.
38.Baranovsky, A. G., Matyushin, V. G., Vlassov, A.
V., Zabara, V. G., Naumov, V. A., Buneva, V. N., and Nevinsky, G. A.
(1997) Biochemistry (Moscow), 62, 1358-1366.
39.Nevinsky, G. A., Breusov, A. A., Baranovsky, A.
G., Prints, A. V., Kanyshkova, T. G., Gal'vita, A. V., Naumov, V. A.,
and Buneva, V. N. (2001) Med. Sci. Monit., 7,
201-211.
40.Andrievskaya, O. A., Buneva, V. N., Zabara, V.
G., Naumov, V. A., Yamkovoi, V. I., and Nevinsky, G. A. (1998) Mol.
Biol. (Moscow), 32, 908-915.
41.Andrievskaya, O. A., Buneva, V. N., Naumov, V.
A., and Nevinsky, G. A. (2000) Med. Sci. Monit., 6,
460-470.
42.Breusov, A. A., Gal'vita, A. V., Benzo, E. S.,
Baranovsky, A. G., Prints, A. V., Naumov, V. A., Buneva, V. N., and
Nevinsky, G. A. (2001) Rus. J. Immunol., 6, 17-28.
43.Kolesnikova, O. P., Kudaeva, O. T., Sukhenko, T.
G., Tuzova, M. N., and Safronova, I. V. (2001) Rus. J. Immunol.,
6, 177-186.
44.Kit, Yu. Ya., Semenov, D. V., Kanyshkova, T. G.,
Kuligina, E. V., Romannikova, I. V., Morozova, O. V., and Richter, V.
A. (1999) Biochemistry (Moscow), 64, 40-46.
45.Popova, N. A. (2000) Immunology [in
Russian], Novosibirsk State University Publishers, Novosibirsk, Pt. 2,
p. 140.