Effects of Coffee Consumption on Oxidative Susceptibility of Low-Density Lipoproteins and Serum Lipid Levels in Humans
G. S. Yukawa1, M. Mune1, H. Otani1, Y. Tone1, X.-M. Liang1, H. Iwahashi2, and W. Sakamoto3*
1Third Department of Internal Medicine, Wakayama Medical University, Wakayama 640, Japan2Department of Chemistry, Wakayama Medical University, Wakayama 640, Japan
3Department of Biochemistry, School of Dentistry, Hokkaido University, Sapporo 060, Japan; fax: +81-11-706-4232; E-mail: sakamoto@den.hokudai.ac.jp
* To whom correspondence should be addressed.
Received April 30, 2003
Since little is known about how coffee intake affects low-density lipoprotein (LDL) oxidative susceptibility and serum lipid levels, we conducted an in vivo study in 11 healthy male students of Wakayama Medical University aged between 20 and 31 years fed an average Japanese diet. On days 1-7 of the study, the subjects drank mineral water. On day 7, the subjects began drinking coffee, 24 g total per day, for one week. This was followed by a one week “washout period” during which mineral water was consumed. Fasting peripheral venous blood samples were taken at the end of each one-week period. LDL oxidation lag time was approximately 8% greater (p < 0.01) after the coffee drinking period than the other periods. Serum levels of total cholesterol and LDL-cholesterol (LDL-C) and malondialdehyde (MDA) as thiobarbituric acid reactive substances (TBARS) were significantly decreased after the coffee drinking period. Finally, regular coffee ingestion may favorably affect cardiovascular risk status by modestly reducing LDL oxidation susceptibility and decreasing LDL-cholesterol and MDA levels.
KEY WORDS: coffee, oxidative susceptibility of LDL, lag time, serum lipids, malondialdehyde
Coffee is one of the most widely consumed psychoactive beverages in our society. It is a well-known and extensively utilized psychotropic agent with effects on mood, cognitive performance, and motor activity. In fact, many investigators have shown that caffeine, one of the main constituents of coffee, has a variety of pharmacological and cellular responses in a wide spectrum of biological systems [1]. These include stimulation of the central nervous system and cardiac muscle, increased urinary output, and relaxation of smooth muscle. In addition to caffeine, coffee also contains a relatively large amount of chlorogenic acid, a tea catechin analog, showing biological effects such as antioxidation, antimutation, anticarcinogenesis, antibiotic, antihypercholesterolemia, antihypertension, and anti-inflammatory actions [2]. Accordingly, coffee must be a useful beverage for health. In contrast, some investigators have reported that caffeine and/or heavy coffee intake may serve as risk factors for lifestyle-related diseases including heart disease and osteoporosis as well as periodontal diseases [3, 4], which are intimately associated with nutrition, exercise, alcohol, smoking, and several other lifestyle factors.
It is currently believed that oxidative modification of low-density lipoproteins (LDL) by free radicals is a key early event in the pathogenesis of atherosclerosis [5]. The rapid uptake of oxidatively modified LDL via a scavenger receptor leads to the formation of foam cells, and oxidized LDL also has a number of other atherogenic properties [5]. Several dietary antioxidants have been shown to inhibit the oxidative modification of LDL [6-8] and, hence, have the potential to decrease LDL oxidation and atherogenesis in vivo [9, 10]. However, the efficiency of the protection of LDL in vivo will depend on several factors, including the absorption of the antioxidants and how they interact with lipoproteins. A number of mechanisms are likely to contribute to inhibition of LDL oxidation by antioxidants.
However, little information is available with regard to the absorption or metabolism of coffee [11-13]. In view of this, the aim of the present study was to investigate the in vivo effects of coffee on the oxidative modification of LDL and lipid metabolism.
MATERIALS AND METHODS
Study design. The study was carried out in accordance with the guidelines of the ethics committee of Wakayama Medical University. The subjects gave written informed consent. To assess the effects of chronic coffee consumption, coffee was consumed by eleven healthy male students (21 and 31 years of age) from Wakayama Medical University. They ate usual Japanese diets and drank only mineral water as a beverage throughout this study. On days 1-7 before the study (Baseline; B), the subjects drank only mineral water as a beverage. Starting on day 7 (After one week; A), subjects drank 150 ml coffee three times per day for one week. This was followed by a one-week (W) “washout” period during which only mineral water was consumed. Fasting peripheral venous blood samples (at least 12 h after coffee ingestion) were taken at the end of each one-week period.
Coffee preparation. Coffee was made by dissolving 8 g of Arabica coffee (Ajinomoto General Foods, Inc., Japan) in one mug of boiling water immediately before drinking. Standard mugs (volume of beverage ingested, 150 ml) were used throughout the study.
LDL oxidation. Serum was obtained from blood samples containing 1 mM EDTA by centrifugation at 1500g for 15 min at 4°C. LDL was isolated from the plasma by rapid ultracentrifugation and oxidized as previously described by Esterbauer et al. [14]. The protein concentration was standardized to 50 mg/liter and oxidation was catalyzed by copper (2 µmol/liter) at 37°C. Conjugated diene formation was monitored at lambda = 234 nm. The lag phase prior to the onset of rapid LDL oxidation is seen as a slope with a slow increase in absorbance. The duration of the lag phase is indicative of the resistance to oxidation of the LDL being examined.
Biochemical analysis. Blood and urine chemistry were carried out by a standard laboratory method using a 7170 autoanalyzer (Hitachi Co., Japan). Malondialdehyde (MDA) concentrations were measured as TBARS using a fluorimetric method.
Assay of caffeine. Caffeine was determined using the method previously described [15]. One milliliter of serum was applied to an Octadecyl Speedisk column (10 µm, Baker Inc., USA), and caffeine was extracted with 400 µl of methanol. Prior to determination of caffeine by HPLC (ODS-120 T column, column size 0.46 x 25 cm, Toyo Soda Co., Japan), extracted samples were diluted 10 times with the mobile phase consisting of CH3OH/0.1% H3PO4 (3 : 7). HPLC analysis was carried out with the mobile phase at flow rate of 1.0 ml/min and detection at 260 nm.
Assay of chlorogenic acid in urine. Urinary chlorogenic acid of three samples was determined by HPLC as described previously [16]. Following extraction from urine samples (900 µl) with 1900 µl of ethyl acetate-methanol (2 : 1 v/v), chlorogenic acid was separated using a C18 column (ODS-120T; column size 0.46 ± 25 cm, Toyo Soda Co.) with 10% acetonitrile in 20 mM phosphate buffer (pH 2.4). The flow rate was 1.0 ml/min, and chlorogenic acid was detected at 325 nm.
Statistical analysis. For statistical calculation, Student's t-test was used. Data are given as mean and standard deviations (mean ± SD). A significant difference was accepted when p < 0.05.
RESULTS
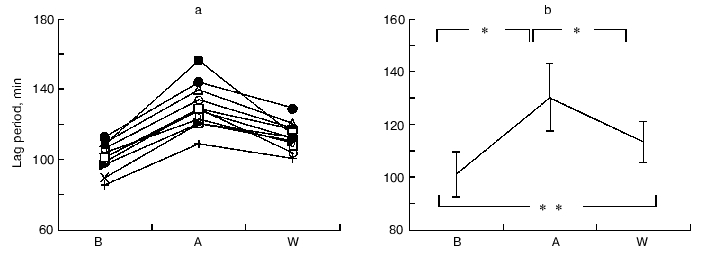
Susceptibility of LDL to oxidation. There was a significant increase in the lag time as the susceptibility of LDL to oxidation before and after coffee consumption (101.0 ± 8.4 min in B, 130.0 ± 12.9 min in A, and 113.3 ± 7.8 min in W, respectively) (Fig. 1).
Serum lipid levels. Throughout the study after coffee consumption, serum lipids, including total cholesterol (TC), LDL-cholesterol (LDL-C), and MDA decreased significantly while triglycerides (TG), HDL cholesterol (HDL-C), and lipoprotein a (Lp(a)) did not change significantly (Table 1). These changes continued at one week after coffee consumption.Fig. 1. Changes in LDL lag time levels at baseline (B), after one week coffee consumption (A) and one week washout period after coffee consumption (W). a) Changes in individuals; b) mean of 11 observations ± SD; * p < 0.001; ** p < 0.005.
Table 1. Serum lipid levels at baseline (B),
after one week coffee consumption (A) and one week washout period after
coffee consumption (W)
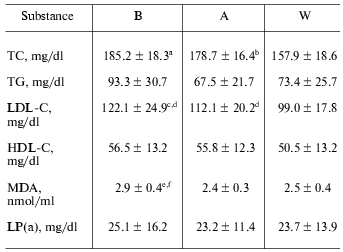
Note: Values are means ± SD. TC, total cholesterol; TG,
triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C,
high-density lipoprotein cholesterol; MDA, malondialdehyde; Lp(a),
lipoprotein a.
a p < 0.0005 compared with W.
b p < 0.002 compared with W.
c p < 0.005 compared with W.
d p < 0.05 compared with B and W.
e p < 0.005 compared with A.
f p < 0.005 compared with W.
Other laboratory data. Throughout the study, there were no significant changes regarding other blood and urine chemistries (Table 2).
Table 2. Laboratory data at baseline (B),
after one week coffee consumption (A), and one week washout period
after coffee consumption (W)
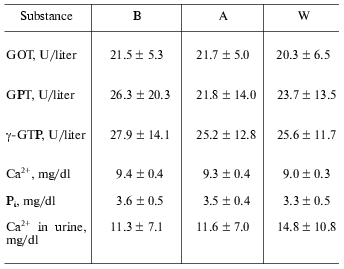
Note: Values are means ± SD. GOT, aspartate aminotransferase;
GPT, alanine-amino transpeptidase; gamma-GTP,
gamma-glutamyl transpeptidase.
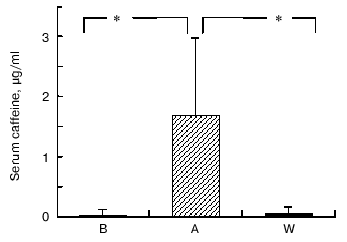
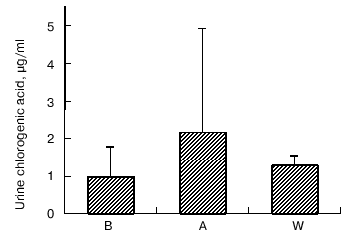
Caffeine and chlorogenic acid concentrations. As shown in Fig. 2, coffee consumption led to an increase in serum caffeine concentration from 0.31 ± 0.03 µg/ml in B, 1.68 ± 0.03 µg/ml in A, and 0.51 ± 0.03 µg/ml in W, respectively. Chlorogenic acid was not detected in serum (data not shown). On the other hand, urine chlorogenic acid concentrations did not show significant changes (1.01 ± 0.76 µg/ml in B, 2.18 ± 2.74 µg/ml in A, and 1.30 ± 0.23 µg/ml in W, respectively) (Fig. 3).
Fig. 2. Changes in serum caffeine concentrations at baseline (B), after one week coffee consumption (A) and one week washout period after coffee consumption (W). Mean of 11 observations ± SD; * p < 0.002.
Fig. 3. Changes in urine chlorogenic acid concentrations at baseline (B), after one week coffee consumption (A) and one week washout period after coffee consumption (W). Mean of three observations ± SD.
DISCUSSION
The effects of coffee on health have been debated throughout the last four centuries since the Arab Abdelkader first wrote about the drink in 1587. Recently, many investigators have suggested that polyphenols as antioxidants in Japanese tea appear to protect the human body against cardiovascular disease, liver disease, and malignancies [2]. Regarding antioxidants, coffee contains chlorogenic acid and caffeic acid, common constituents of coffee. Therefore, coffee should also be expected to be a useful beverage for human health. According to the report of Cooper et al. [17], the median daily caffeine consumption was estimated to be 210 mg (range 0-849.6 mg) from intakes of coffee, tea, and other caffeinated beverages. According to the report of McAnlis et al. [18], coffee did not affect serum lipid levels and susceptibility of LDL to oxidation in humans. The object of this study was, therefore, to assess whether consumption of relatively large amounts of coffee and the control of the beverage over a week affected serum lipid levels and/or protected the LDL from oxidation. According to the data from serum caffeine concentrations of the present study, this study design is appropriate for investigating the effect of coffee consumption on serum lipids and in vitro LDL susceptibility. In this study, coffee ingestion resulted in a significant decrease in serum levels of cholesterol, LDL cholesterol, and MDA, and a significant decrease in susceptibility of LDL to oxidation, indicating that coffee consumption might protect against atherosclerosis due to lowering serum lipid levels and improving LDL susceptibility. Although the reasons for discrepancies compared with a few previous reports [18, 19] are not yet clear, it may be due to the difference of the study design, especially relatively large amounts of coffee consumption and the strictly controlled beverage intake in our study. Recently, however, a report consistent with our results was published [20]. The addition of increasing concentrations of coffee had a very highly significant dose dependant effect on the LDL oxidation [21], suggesting that the antioxidants present in coffee if absorbed in large enough quantities should be able to protect the LDL from oxidation in vivo. Subsequent studies were carried out to see whether or not compounds in coffee are absorbed into the serum at high enough quantities to protect the LDL from oxidative modification in vivo.
There is, however, very little known about coffee absorption and metabolism in vivo [11-13], and the extent of absorption of coffee remains an important unsolved problem in judging its potential health effects. In this study, chlorogenic acid, one of the major antioxidants of coffee, was not detected in serum and did not change in parallel with coffee consumption in urine, demonstrating that plasma chlorogenic acid might be present as a conjugated form with glutathione [16]. This study also showed urinary chlorogenic acid concentrations did not change after coffee consumption, indicating that chlorogenic acid is present in the diet as part of fruits and vegetables [16].
In summary, it therefore seems likely that any cardioprotective effects of coffee are a reduction of serum lipid and MDA levels, and a decrease in the susceptibility of LDL to oxidation. However, large-scale controlled studies, including age, sex, race, and lifestyle will be required to resolve whether coffee is a useful beverage for human health.
REFERENCES
1.Dews, P. B. (1982) Ann. Rev. Nutr.,
2, 323-341.
2.Sugiyama, K., He, P., Wada, S., and Saeki, S.
(1999) J. Nutr., 129, 1361-1367.
3.Kamagata-Kiyoura, Y., Ohta, M., Cheuk, G., Yazdani,
M., Saltzman, M. J., and Nakamoto, T. (1999) J. Periodontol.,
70, 283-288.
4.Sesso, H. D., Gaziano, J. M., Buring, J. E., and
Hennekens, C. H. (1999) Am. J. Epidemiol., 149,
162-167.
5.Goldstein, J. L., Ho, Y. K., Basu, S. K., and
Brown, M. S. (1979) Proc. Natl. Acad. Sci. USA, 76,
333-337.
6.DeWhalley, C. V., Rankin, S. M., Hoult, J. R.,
Jessup, W., and Leake, D. S. (1990) Biochem. Pharmacol.,
39, 1743-1750.
7.Frankel, E. N., Kanner, J., German, J. B., Parks,
E., and Kinsella, J. E. (1993) Lancet, 341, 454-457.
8.Fuhrman, B., Lavy, A., and Aviram, M. (1995) Am.
J. Clin. Nutr., 61, 549-554.
9.Hertog, M. G., Kromhout, D., Aravanis, C.,
Blackburn, H., Buzina, R., Fidanza, F., Giampaoli, S., Jansen, A.,
Menotti, A., and Nedeljkovic, S. (1995) Arch. Int. Med.,
155, 381-386.
10.Knekt, P., Jarvinen, R., Reunanen, A., and
Maatela, J. (1996) Brit. Med. J., 312, 478-481.
11.Scalbert, A., and Williamson, G. (2000) J.
Nutr., 130 (8S Suppl.), 2073S-2085S.
12.Carrillo, J. A., and Benitez, J. (2000) Clin.
Pharmacokinet., 39, 127-153.
13.Olthof, M. R., Hollman, P. C., and Katan, M. B.
(2001) J. Nutr., 131, 66-71.
14.Esterbauer, H., Striegl, G., Puhl, H., and
Rotheneder, M. (1989) Free Rad. Res. Commun., 6,
67-75.
15.Sakamoto, W., Nishihira, J., Fujie, K., Mizuno,
S., Ozaki, M., and Yukawa, S. (2000) J. Nutr. Sci. Vitaminol.,
46, 316-320.
16.Sakamoto, W., Isomura, H., Fujie, K., Nishihira,
J., Ozaki, M., and Yukawa, S. (2003) Toxicology, 183,
255-263.
17.Cooper, C., Atkinson, E. J., Wahner, H. W.,
O'Fallon, W. M., Riggs, B. L., Judd, H. L., and Melton, L. J. (1992)
J. Bone Miner. Res., 7, 465-471.
18.McAnlis, G. T., McEneny, J., Pearce, J., and
Young, I. S. (1998) Eur. J. Clin. Nutr., 52, 202-206.
19.Miyake, Y., Kono, S., Nishiwaki, M., Hamada, H.,
Nishikawa, H., Koga, H., and Ogawa, S. (1999) Ann. Epidemiol.,
9, 121-126.
20.Christensen, B., Mosdol, A., Retterstol, L.,
Landaas, S., and Thelle, D. S. (2001) Am. J. Clin. Nutr.,
74, 302-307.
21.Richelle, M., Tavazzi, I., and Offord, E. (2001)
J. Agr. Food Chem., 49, 3438-3442.