REVIEW: Photobiological Principles of Therapeutic Applications of Laser Radiation
Yu. A. Vladimirov*, A. N. Osipov, and G. I. Klebanov
Department of Biophysics, Russian State Medical University, ul. Ostrovityanova 1, Moscow 117513, Russia; fax: (7-095) 246-4630; E-mail: yuvlad@newmail.ru* To whom correspondence should be addressed.
Received September 30, 2003
Laser therapy based on the stimulating and healing action of light of low-intensity lasers (LIL), along with laser surgery and photodynamic therapy, has been lately widely applied in the irradiation of human tissues in the absence of exogenous photosensitizers. Besides LIL, light-emitting diodes are used in phototherapy (photobiostimulation) whose action, like that of LIL, depends on the radiation wavelength, dose, and distribution of light intensity in time but, according to all available data, does not depend on the coherence of radiation.
KEY WORDS: laser radiation, phagocytes, heme protein
The action of visible light on the specialized cells of the eye underlies sight. Nevertheless, the problem of the possibility of action of visible light on other human and animal cells and tissues was for a long time left unexplored. The appearance of lasers, sources of intensive visible and infrared radiation, gave a new impulse to this problem (see reviews [1, 2]). Lasers became widely used in surgery and therapy, and the question of whether visible light has an effect was solved by itself. The mechanism of action of laser light can, nevertheless, vary in different situations and for the most part is poorly studied.
Activation of life processes under laser radiation, often called “biostimulation”, is of most interest [3-6]. Under large doses of laser radiation, its positive action changes, as a rule, into inhibition of vital activity processes, which is a main hindrance to a successful application of laser therapy and a cause of disappointment.
Nevertheless, radiation by low-intensity lasers (LIL) and light-emitting diodes (LED) is widely used by physical therapists and dentists (to reduce pain), dermatologists (treatment of edema, eczema, and dermatitis), surgeons (treatment of persisting ulcers, burns, “diabetic foot”), rheumatologists (either to relieve pain or treat chronic diseases--arthritis and arthrosis), therapeutists, in veterinary medicine, sports medicine, and rehabilitation centers [1, 2, 7, 8]. According to Medline data (search by key words laser and therapy), 1700 to 2400 papers on therapeutic application of lasers have been published annually during the last ten years, this number growing steadily.
Exposure to lasers as well as LED light is currently applied in therapy. The most effective irradiation is that in the red and near infrared range of the spectrum. The most commonly used sources are the helium-neon laser (He-Ne) (radiation at 632.8 nm), the gallium-aluminum laser (Ga-Al) (630-685 nm), the helium-neon-arsenate laser (He-Ne-As) (780-870 nm), and the gallium-arsenate laser (Ga-As) (904 nm), as well as light emitting diodes whose emission band lies in a wide region of the spectrum (670 to 950 nm) [2]. The main reason for using the sources radiating in the red and near infrared spectral region is the fact that hemoglobin does not absorb in this region and light can penetrate deep into living tissue.
Comparison of the therapeutic effects of a coherent (LIL) and an incoherent (LED) source showed no significant difference [2, 9, 10]. This is also relevant to the action of light on the level of cells where coherent and incoherent sources had the same effect at the same wavelengths, intensities, and radiation times [11, 12].
ACTION OF VISIBLE LIGHT ON ANIMAL CELLS AND TISSUES
What is it known about the action of laser radiation on objects simpler than an ill human being? Consider some well-established facts.
Radiation with wavelength of 400 to 500 nm and about 600 nm brought about accelerated cell division in various microorganisms and enhanced protein synthesis. For instance, radiation of two types of fibroblasts with a He-Ne laser for 15 min (1 W/m2) accelerated the growth of the population in the exponential phase and attachment of cells to substrate [13]. Radiation of L-cells with a He-Ne laser resulted in an increased mitotic index on the 3rd and 4th day after irradiation [14]. A small but reliable increase of the mitotic index was also observed after a single radiation of retina epithelium cells with a He-Ne laser (83 W/m2, for 10 min) [15]. Generally, an expressed extreme relationship between the stimulating action of laser radiation and dose is observed. The interval of the intensities within which a positive effect was observed was 1.5 to 2 orders of magnitude [16, 17]. For instance, almost twice as great growth stimulation was observed on the radiation of Escherichia coli with an infrared laser at 890 nm at a radiation dose of 0.7 J/m2, and inhibition of the growth took place at a dose of above 2.5 J/m2 [17].
Radiation of isolated liver mitochondria with a He-Ne laser brought about enhanced ATP-ADP metabolism [18], an increased content of ATP, a growth of the electric potential across inner membranes and pH in matrix, as well as small changes in the matrix configuration [19]. Evident morphological changes in lymphocyte mitochondria were observed after radiation of those cells with a He-Ne laser [20].
Radiation with a He-Ne laser does not generally bring about the blast transformation of isolated lymphocytes but enhances significantly the blast transformation brought about by phytohemagglutinin (PHA), and also leads to an increase of the mitotic index on the 3rd and 4th day after radiation with low doses [20]. The action of He-Ne radiation brings about an increased content of intracellular calcium in leukocytes, an enhanced dyeing of nucleic acids in cells by the fluorescent dye acridine orange, and changes in the morphological structure of chromatin. The action of laser radiation in optimal dose was similar by all parameters to the action of PHA (the data and bibliography are presented in review [20]).
Laser radiation also had an effect on phagocyte cells both in vivo and in vitro [21]. Below, we shall consider the data obtained in our laboratory.
On radiation of blood immediately in blood vessels, vasodilatation was observed above and below the site of radiation, the effect disappearing on the substitution of blood for perfused physiologic saline [22]. The effect of vasodilatation results in the improvement of microcirculation [23] and blood supply in organs [24].
Thus, the effects observed in clinic--the antiinflammatory action of laser radiation, an accelerated regeneration of damaged tissues, and the improvement of blood circulation in organs--can be associated with the lasers' effects obtained in experiments: 1) growth of the activity of certain cells such as leukocytes and phagocytes, as well as an increased content of calcium ions in the cytoplasm of these cells; 2) enhancement of cell division and cell growth; 3) activation of the synthesis of proteins and cytokines, and 4) improvement of blood circulation in the bloodstream due to the relaxation of vessel walls (vasodilatation).
EARLY HYPOTHESES OF THE MECHANISMS OF ACTION OF LASER
RADIATION
There exist a great number of hypotheses of possible mechanisms of laser radiation. They can be divided into two categories.
1. Hypotheses based on the idea of a specific action of coherent (laser) radiation on human and animal tissues, biological structures as a whole, water structure, etc.
2. Hypotheses of the photochemical action of light, including the radiation of lasers, LEDs, and other sources of visible and near infrared light. Karu noted the following hypotheses concerning the mechanism of action of laser radiation.
First, this is the “singlet oxygen” hypothesis (1981), according to which [25] the light-absorbing molecules such as porphyrins and flavoproteins can be changed, for example, in the respiratory chain of mitochondria into derivatives possessing the properties of photosensitizers [26]. Under the action of light, these compounds evolve singlet oxygen that can stimulate, in turn, such processes as the synthesis of RNA and DNA. The authors of the hypothesis found corroboration of this idea in the fact that the spectra of activation of the synthesis of those compounds in HeLa cell cultures contained peaks that could be ascribed to porphyrins and flavin compounds [11, 25]. The possibility of formation of singlet oxygen under radiation is also considered in paper [27].
Second, the hypothesis of laser light action on the oxidation-reduction properties of electron carriers suggested in 1988 [28]. The excitation in cytochrome-oxidase complex of such chromophores as CuA, CuB, or hemes a(a3) influences the oxidation-reduction state of these centers and, consequently, the electron transfer rate in the molecule [28].
THE HYPOTHESIS OF THREE MECHANISMS OF ACTION OF LASER
RADIATION
In 1994, on the basis of the analysis of literature data available at that time and our own experiments, one of us formulated the hypothesis of three mechanisms of action of low-intensity laser radiation on human cells and the human body [29], according to which the biological action of laser radiation in the visible region of light and its clinical application is based on three reactions: 1) photodynamic action on membranes accompanied by intracellular calcium increase and cell stimulation; 2) photoreactivation of Cu-Zn superoxide dismutase; and 3) photolysis of the metal complexes of NO with release of this vasodilator. It was postulated that these three effects underlie the indirect bactericidal, regenerative, and vasodilatation action of laser radiation ([29], p. 63). The experimental data corroborating this hypothesis are presented below.
ACCELERATED WOUND HEALING ON LASER IRRADIATION
The phenomenon of photoreactivation of cellular superoxide dismutase (Cu-Zn-SOD) was discovered as the result of the analysis of the data obtained in studies of the action of He-Ne laser on wound healing.
As already stated, a positive action of laser radiation is observed when using it for treatment of persistent wounds and trophic ulcers; in particular, radiation of wounds with a He-Ne laser resulted in accelerated wound healing in children [30]. Probably, it is partially associated with the intensification of antiradical protection in the wound area. This could be judged of from experiments demonstrating changes in the chemiluminescence intensities of wound exudates in the presence of added hydrogen peroxide. The luminescence was associated with the formation of superoxide radical in the process of decomposition of hydrogen peroxide by exudate: the addition to exudate of superoxide dismutase (SOD) removing superoxide radicals, as well as the addition of catalase removing hydrogen peroxide, suppressed the flash of chemiluminescence. Radiation of exudate with a He-Ne laser also suppressed the luminescence, the laser light thus acting as catalase or superoxide dismutase. It would be natural to suggest that the activity of catalase or superoxide dismutase in exudate was initially reduced under some conditions and laser radiation reactivated one of those enzymes. It should be noted that both enzymes absorb at the He-Ne laser light wavelength of 633 nm.
PHOTOREACTIVATION OF Cu-Zn SOD UNDER THE ACTION OF He-Ne
LASER
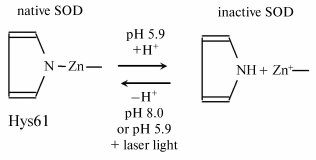
It was shown in experiments with isolated erythrocyte Cu-Zn-SOD that a decreased pH of the solution and hydrogen peroxide inactivated the enzyme, while its complete reactivation was observed under subsequent irradiation of the inactivated enzyme at pH 5.9 with He-Ne laser light [31, 32]. Such a phenomenon can probably be observed in living organisms. If the acidity of the medium decreases at a locus of the human body (which is typical of foci of impaired vital functions of cells), the activity of SOD at the locus can be expected to decrease due to low pH.
To elucidate the mechanism of inactivation and photoreactivation of SOD in acid medium, the absorption spectra and EPR signals of the enzyme were investigated on its acid inactivation and after laser irradiation [31, 32]. All these data suggest that protonation of the histidine residue in the active center of the enzyme underlies the inactivation of Cu-Zn-SOD, and radiation brings about the deprotonation of histidine and formation of the >N-Zn bond to restore the active center structure and activity of the enzyme (see scheme).
Simultaneously, other properties of the active enzyme are restored--its absorption spectrum and EPR spectrum.
Scheme
Deprotonation under the action of light is not a special property of histidine in the active center of Cu-Zn-SOD. The dissociation constant of many chromophores, including aromatic amino acids, increases when the molecule becomes electronically excited [33]. It can be considered that the pK value of the Cu-His complex is above 5.9 in the excited electron state; thus, this complex will be deprotonated under intensive radiation.
POSSIBLE BIOLOGICAL CONSEQUENCES OF INACTIVATION AND REACTIVATION
OF SOD
Superoxide dismutase reduces the concentration of superoxide radical O2- catalyzing the reaction of dismutation:
O2- + O2- --> O2 + H2O2.
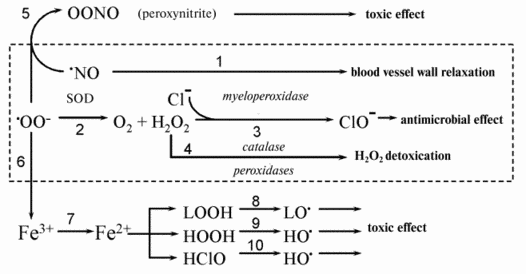
As a result, the stationary superoxide concentration decreases in cells and tissues where this radical constantly forms especially under pathologic conditions. Figure 1 shows a scheme demonstrating the metabolism of superoxide radical which demonstrates why excess radical can be toxic for cells and tissues.
Two reactions appear to underlie the pathogenic action of O2-: formation of ferrous iron ions (from ferric ones) (reaction (7) in Fig. 1), and binding of nitric oxide (reaction (5)). The protective action of SOD is due to a decrease in the concentration of superoxide radicals O2- in the medium as a result of their dismutation (reaction (2) in Fig. 1) with the subsequent removal of hydrogen peroxide in the reactions catalyzed by catalase and glutathione peroxidase.Fig. 1. Possible mechanisms of the cytotoxic action of superoxide radical. See text for explanations.
The first cause of the pathogenic action of superoxide excess is the release of free ferrous iron from different depots. Free ferric iron is apparently absent in tissues but it is present in cells in the form of ferritin. Penetrating into ferritin, O2- reduces ferric iron to a free ferrous ion (reaction (7) in Fig. 1). Fe2+ ions participate in at least three reactions accompanied by the formation of new free radicals (Fig. 1): the reaction with hydrogen peroxide (Fenton reaction (9)), reaction with hypochlorite (10), and reaction with lipid hydroperoxides (8). The Fenton reaction results in the formation of hydroxyl radical, which is highly reactive. Its interaction with proteins brings about their denaturation, and its reactions with nucleic acids are accompanied by mutagenic and lethal effects (see review [34]). It was shown in the investigations by A. N. Osipov and coworkers carried out with the method of spin traps that hydroxyl radical is also formed when Fe2+ interacts with hypochlorite and apparently with a greater yield compared to the reaction of Fe2+ with H2O2 [35]. Reactions (9) and (10) in Fig. 1 occur mainly in the aqueous phase of living cells and tissues where hydrogen peroxide and hypochlorite are dissolved. Fe2+ ions in the lipid phase of biological membranes and lipoproteins act as potent catalysts (better to say cofactors) of lipid free radical chain oxidation. Their prooxidant action is based on the reaction of oxidation chain branching in the interaction of Fe2+ with unsaturated fatty acid hydroperoxides (reaction (8) in Fig. 1) (see reviews [36-38]).
The totality of reactions (7)-(10) underlies a mediated cytotoxic and mutagenic action of superoxide radical O2-.
The second cause of a harmful action of superoxide radical excess is the interaction of the radical with nitric oxide NO* (reaction (5)). Many cells such as, for example, endothelial cells, blood neutrophils, and tissue macrophages are able to simultaneously release superoxide radicals and produce nitric oxide. Meanwhile, superoxide and NO* interact with each other actively to form peroxynitrite-anion with the subsequent formation of hydroxyl radical [39]. Peroxynitrite has an extremely potent cytotoxic effect. Removing one of the components of the reaction of peroxynitrite formation (reaction (5)), SOD protects tissues from damage and prevents the destruction of the relaxation factor thus reducing cell damage in many pathological states. Review [29] gives examples of the beneficial action of SOD. As an example we can mention decreased radical formation and myocardial damage on heart ischemia-reperfusion [43].
Damage to heart and brain due to oxygen deficiency in certain areas of their tissues is a main cause of human death in developed countries. It might seem that restoration of blood circulation must decrease or prevent damage to cells and tissues suffering oxygen deficiency. Nevertheless, McCord and coworkers showed as long ago as 1980s that reperfusion may cause additional damage to tissue (reoxygenation damage) which to a significant extent is due to the additional formation of active oxygen forms, in particular, superoxide [40-44]. Thus, on reperfusion of a heart that has been some time in the state of ischemia (absence of blood circulation) abrupt aggravation of the state of the organ occurs, which manifests itself mainly by fibrillation. Introduction of superoxide dismutase into the perfusion liquid reduces this effect significantly; thus, it can be concluded that it is free radicals that are mainly responsible for the reoxygenation damage to tissue. It was shown in the works by V. A. Monich and coworkers that if irradiation of the heart region in laboratory animals with intensive light is carried out instead of SOD injection, reperfusion heart damage and fibrillation are removed [45]. Simultaneously, formation of lipid peroxidation products in tissues is reduced to the level of intact tissue [46]. The authors explained these effects by the phenomenon of SOD reactivation under the action of light.
PHOTODYNAMIC ACTION OF ENDOGENOUS SENSITIZERS
If a small amount of sensitizer, for example, hematoporphyrin or phthalocyanine, is added to phospholipids, lipid oxidation takes place under the action of light (photodynamic effect) and peroxide formation occurs (photoperoxidation) [47]. As it is known, natural sensitizers, first of all hematoporphyrin and its derivatives, are accumulated in cell membranes in certain diseases. The action of lipid peroxidation on the phospholipid layer of membranes is well investigated and comes to several main effects [37]: permeability to H+ and/or OH- ions enhances selectively; permeability to Ca2+ ions increases; electrical stability decreases, which can bring about a “self-breakdown” of membranes by their own electric potential. In cell membranes, this is added to oxidation of SH-groups and damage to the calcium pump (Ca2+-ATPase) that becomes a channel for Ca2+ ions. Besides, calcium ions start “leaking” along the channel formed instead of the pumping in the direction of their lower concentration [48, 49], i.e., to hyaloplasm. One of the main results of all these effects is an increased intracellular Ca2+ concentration. In fact, an increased content of intracellular calcium is observed under irradiation of such cells as lymphocytes and granulocytes with He-Ne laser light [47], which results, in turn, in cell activation and/or proliferation.
It can be supposed that the sequence of events under laser irradiation in this case and in many other cases looks like: 1) absorption of a photon by an endogenous photosensitizer followed by lipid peroxidation (photoperoxidation); 2) entering of calcium ions into cells; 3) activation of intracellular processes.
Biological consequences of increased Ca2+ concentration in cytoplasm will differ depending on the type of cells exposed to laser radiation. For example, in blood irradiation, the action of radiation on leukocytes (neutrophils and monocytes) protecting the organism against microbes and participating in regulation of blood circulation is significant. It has been shown that lipid peroxidation products and oxidized blood plasma lipoproteins induce prestimulation (priming) of phagocyte cells, i.e., two- or threefold enhancement of their generation of active oxygen species in response to a stimulus such as bacterial cell membranes, lectins (for example, phytohemagglutinin), calcium-carrying antibiotics, and some other substances. The essence of the phenomenon of prestimulation (priming) is considered in our publications [50, 51] and consists of the following steps. Initially, a phagocyte cell has on its surface a number of receptors for stimulus under whose action the cell is activated, which is accompanied by the generation of active oxygen species and chemiluminescence in the presence of luminol. If the cell is preliminarily incubated with a compound increasing the amount of calcium ion in the cytoplasm, the number of receptors on the cell surface increases, for example, this is observed when neutrophils are incubated with lipid peroxidation products. Now the addition of a stimulus results in a greater chemiluminescence (CL) response than that of the intact leukocytes.
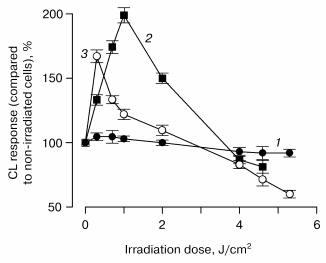
The effect of He-Ne laser radiation on the priming of leukocytes isolated from the blood of healthy persons and patients with bronchitis was investigated. A significant difference was observed in the effects of laser radiation on blood phagocytes in different groups of patients. In the first group, laser radiation had no effect on leukocytes since the cells of those patients apparently contained no endogenous sensitizer. Radiation of the cells by laser light in the presence of 10 nM added phthalocyanine sensitizer resulted in a significant effect of prestimulation at low radiation doses. In the second group, a small prestimulation was demonstrated in the absence of an exogenous sensitizer although the effect was greater and observed at lower doses if a sensitizer was added. In the third group, the effect of prestimulation was significant in the absence of exogenous sensitizers, and it weakened in their presence. The general conclusion is that leukocytes from the blood of different patients varied only in the initial content of endogenous sensitizer; in other aspects, the action of He-Ne laser was similar: priming was observed at low doses and suppression of cell reactivity was observed at high doses (i.e., CL response to stimulus introduction). Subsequent experiments showed that the irradiation effect depended on the amount of hematoporphyrin in the patients' blood plasma: the larger the amount of porphyrin the lower was the dose of laser radiation at which the effect of priming was observed.
This is seen in Fig. 2, which shows the relationships between the irradiation doses and CL responses of leukocytes incubated in the presence of 10 µl of blood plasma of patients with bronchopulmonary diseases. It is seen that the greater amount of porphyrin in 10 µl of plasma, the less is the radiation dose at which the CL response of cells enhances. Therefore, hematoporphyrin is a most probable natural photosensitizer of laser light.
Fig. 2. Relationship between the chemiluminescence response of leukocytes incubated in the presence of 10 µl of blood plasma of patients with bronchopulmonary diseases and a dose of radiation with He-Ne-laser: 1) control; 2) 4.1 pmol porphyrin in 10 µl of blood plasma; 3) 8.5 pmol porphyrin in 10 µl of blood plasma.
ACTIVATION OF PROTEIN SYNTHESIS
An immediate biological consequence of prestimulation of leukocytes (granulocytes and monocytes) by laser irradiation of blood is activation of phagocytosis, i.e., the destruction of bacteria and fungi by these cells. This direct and thus short-term effect of laser irradiation is significantly supplemented by further consequences associated with activation of biosynthetic processes in irradiated cells. It was shown that laser irradiation of cells was able to induce the synthesis of a number of proteins. For example, it turned out that the activity of superoxide dismutase was enhanced upon laser irradiation of leukocytes, this effect being suppressed by the protein synthesis inhibitor cycloheximide [52]. Induction of the synthesis of another protein, inducible NO-synthase, by laser light was also of great interest. It was shown that after irradiation peritoneal macrophages started synthesizing NO, which was detected as NO2- production. Cycloheximide suppressed that effect too [52].
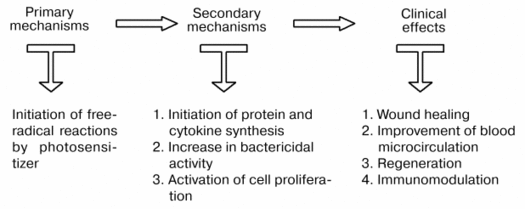
Therefore, a beneficial action of laser irradiation is the result of initiation of primary, free-radical reactions inducing activation of cells (leukocytes, fibroblasts, keratinocytes, etc.) which is expressed in increased bactericidal activity, production of proteins and cytokines, and cell proliferation. All these events are the basis of the therapeutic action of laser therapy (Fig. 3).
Fig. 3. Scheme demonstrating relations between the primary and secondary mechanisms of action of laser radiation.
PHOTOLYSIS OF NITROSYL COMPLEXES OF HEME PROTEINS
Below we consider the third hypothesis of the possible mechanism of action of a low-intensity laser radiation--photolysis of nitrosyl complexes of heme proteins. Soon after the hypothesis was formulated, we demonstrated the photosensitivity of NO complexes with hemoglobin and cytochrome c. EPR and spectrophotometric methods were used. It was shown that the NO-hemoglobin EPR signal decreased under the action of laser radiation on NO-hemoglobin. Simultaneously, nitric oxide is released, as can be judged from the increasing EPR signal of NO spin adduct. Apparently, decomposition of paramagnetic complexes brings about the appearance of free NO, according to the following reaction:
hnu + HbNO --> Hb(Fe2+) + NO.
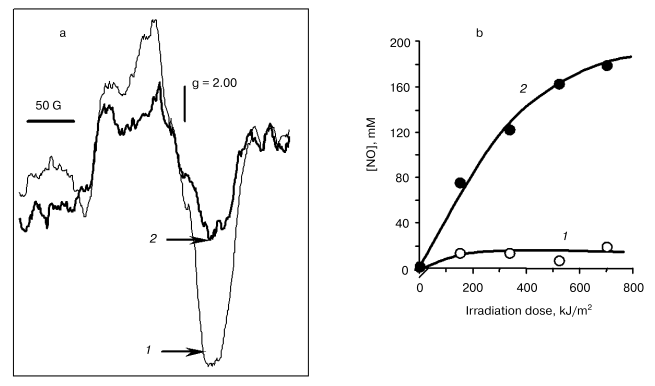
The EPR spectrum of the cytochrome c (Fe2+)-NO complex is a singlet asymmetric line with a g-factor of 2.00 and a half-width of ~100 G (Fig. 4a). Under the action of laser light, the EPR signal amplitude decreases, this being faster the higher the power of irradiation.
As shown by experiments with the use of spin traps (Fig. 4b), the release of nitric oxide takes place as well. Thus, on irradiation of nitrosyl complexes of cytochrome c (cyt c) photolysis of the complex takes place with the release of free NO:Fig. 4. Action of irradiation on the intensity of the EPR signal of cytochrome c nitrosyl complexes (a) and formation of free NO against dose of radiation with He-Cd laser (b): 1) control; 2) radiation 8 kJ/m2.
hnu + cyt c-NO --> cyt c + NO*.
ACTIVATION OF ELECTRON TRANSFER IN MITOCHONDRIA UNDER LASER
IRRADIATION
Irradiation of isolated liver mitochondria with He-Ne laser light resulted in an increase in the content of ATP, transmembrane potential, and DeltapH and changes in the ultrastructure of organelles. All these observations suggest the improvement of respiratory chain functioning. An increased level of ATP in cells was observed also after irradiation of human blood lymphocytes with the light of an infrared (diode) laser at radiation wavelength of 820 nm. These changes in mitochondria indicate the preparation of the cell for division, though it is not clear whether they precede other changes in the cells caused by laser radiation or follow them.
POSSIBLE ROLE OF PHOTOLYSIS OF NO COMPLEXES WITH CYTOCHROME
OXIDASE
Investigations of action spectra of lasers and light-emitting diodes carried out by T. Karu and coworkers demonstrate the participation of cytochrome oxidase in a stimulating action of light in the red and near infrared range of the spectrum [2, 28, 53].
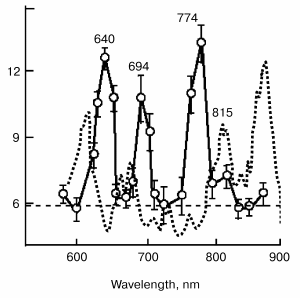
Figure 5 demonstrates the absorption spectra of HeLa cell cultures (dotted line) compared to the action spectra of the synthesis of RNA (points). The action spectra of the activation of the synthesis of RNA and DNA in HeLa cells and adhesion of the plasma membranes of these cells at the stage of exponential growth have four expressed peaks at 630 to 657, 685 to 694, 752 to 774, and 815 to 823 nm. Three of them correspond to the maxima in the absorption spectrum of the cell (616, 676, and 809 nm) and are ascribed to the absorption of the metal-containing complexes of cytochrome oxidase in eukaryotes and, correspondingly, to the complex of cytochromes b-d in bacteria [2, 28, 53, 54].
In our opinion, the differences in the spectra can be explained by the fact that no electron carriers themselves participate in the photobiological effect on cytochrome oxidase level but only their complexes with NO, while light is absorbed by the carriers themselves.Fig. 5. Action spectrum of He-Ne laser radiation on irradiation of mitochondria (results obtained from Karu's work [2]). Figures near the curves designate wavelengths.
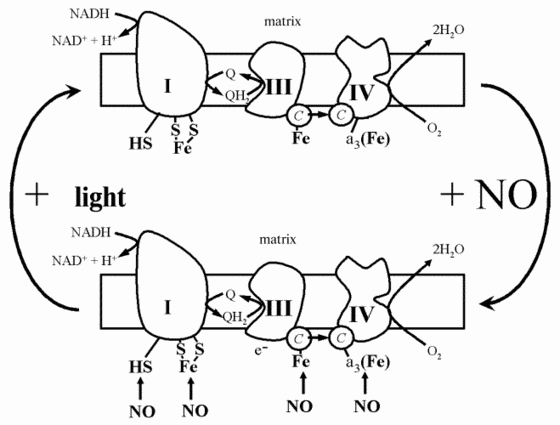
On the basis of all of the data, the following scheme can be presented concerning the action of laser radiation on mitochondria or even whole cells (Fig. 6). Mitochondrial respiration in the absence of light is partially suppressed by nitric oxide synthesized by mitochondrial NO-synthase (Fig. 6). Nitric oxide inhibits respiration due to binding with such electron carriers as cytochromes and cytochrome oxidase, and possibly iron-sulfur complexes. Irradiation by intense light brings about the photolysis of these complexes and restoration of respiration and ATP synthesis (Fig. 6).
Therefore, a beneficial action of the low-intensity laser light, as well as the light of other sources, for example, light-emitting diodes, is based on different primary photochemical reactions, three of them can be considered proved: 1) photoreactivation of Cu-Zn-superoxide dismutase inactivated at low pH in hypoxic foci; 2) the photodynamic action of endogenous sensitizers, first of all hematoporphyrin, whose content in tissues is sensible in healthy persons and can be increased in pathology; and 3) the photolysis of complexes of metal-containing proteins with nitric oxide which brings about the release of free NO and reactivation of respiration carriers.Fig. 6. Scheme demonstrating the action of nitric oxide and laser radiation on the functioning of the mitochondrial electron transfer chain.
Since laser radiation at low doses makes a stimulating effect on cells, and that at high doses a damaging one, a knowledge of the conditions of the course of these reactions in each individual patient (e.g., the content of porphyrins in tissue) is necessary for correct dosing and effective application of light therapy.
The investigations presented in this review were carried out with financial assistance of the Russian Foundation for Basic Research (grant Nos. 03-04-48891 and 03-04-49267).
REFERENCES
1.Tuner, J., and Hode, L. (1999) Low Level Laser
Therapy: Clinical Practice and Scientific Background, Prima Books,
Goengesberg, Sweden.
2.Karu, T. (2003) in Low-Power Laser Therapy,
CRC Press, N. Y., pp. 4825-4841.
3.Karu, T. I., Kalendo, G. S., Letokhov, V. S., and
Lobko, V. V. (1984) Nuovo Cimento D, 3, 309-316.
4.Karu, T. I., Kalendo, G. S., Letokhov, V. S., and
Lobko, V. V. (1984) Nuovo Cimento D, 3, 317-324.
5.Karu, T. I., Pyatibrat, L. V., and Kalendo, G. S.
(1987) Nuovo Cimento D, 6, 1485-1491.
6.Karu, T. (1989) J. Photochem. Photobiol. B,
3, 638-640.
7.Baxter, G. D. (1994) Therapeutic Lasers: Theory
and Practice, Churchill Livingstone, London.
8.Simunovic, Z. (2000) Lasers in Medicine and
Dentistry, Vitgraf, Rijeka.
9.Sazonov, A. M., Romanov, G. A., Portnoi, L. M.,
Odinokova, V. A., Karu, T. I., Lobko, V. V., and Letokhov, V. S. (1985)
Sovetskaya Meditsina, 12, 42-49.
10.Karu, T. I. (1989) Photobiology of Low-Power
Laser Therapy, Harwood Academic Press, London.
11.Karu, T. I., Kalendo, G. S., Letokhov, V. S., and
Lobko, V. V. (1982) Nuovo Cimento D, 1, 828-835.
12.Bertoloni, G., Sacchetto, R., Baro, E.,
Ceccherelli, F., and Jori, G. (1993) J. Photochem. Photobiol. B,
18, 191-196.
13.Boulton, M., and Marchall, J. (1986) Lasers
Life Sci., 1, 125-134.
14.Gamaeva, N. F., Shishko, E. D., and Yanish, V.
(1983) Doklady AN SSSR, 273, 224-227.
15.Yew, D. T., Lam, S. T. L., and Chan, Y. W. (1982)
Acta Radiol. Oncol. Rad. Phys., 21, 433-438.
16.Karu, T. I. (1990) Photochem. Photobiol.,
52, 1089-1998.
17.Tiphlova, O., and Karu, T. (1991) Crit. Rev.
Biomed. Eng., 18, 387-412.
18.Passarella, S., Ostuni, A., Atlante, A., and
Quagliariello, E. (1988) Biochem. Biophys. Res. Commun.,
156, 978-986.
19.Passarella, S., Roncall, L., Cicero, R., and
Quagliariello, E. (1988) Laser Life Sci., 2, 161-165.
20.Karu, T. (1992) Laser Therapy, 4,
5-24.
21.Ricevuti, G., Mazzone, A., Monaia, C., Fratino,
P., Degiulio, R., Dell'Acqua, R., Leonardi, G., Jucci, A., and Sacchi,
S. (1989) Inflammation, 13, 507-527.
22.Schwengel, R. H., Gregory, K. W., Hearne, S. E.,
Scott, H. J., Beauman, G. J., Mergner, W. J., Caplin, J. L., and
Ziskind, A. A. (1993) Lasers Surg. Med., 13, 284-295.
23.Maegawa, Y., Itoh, T., Hosokawa, T., Yaegashi,
K., and Nishi, M. (2000) Lasers Surg. Med., 27,
427-437.
24.Kozlov, V. I., Builin, V. A., Samoilov, N. G.,
and Markov, I. I. (1993) Basis of Laser Physio- and Reflexotherapy
[in Russian], Zdorov'ya, Samara-Kiev.
25.Karu, T. I., Kalendo, G. S., and Letokhov, V. S.
(1981) Lett. Nuovo Cimento, 32, 55.
26.Giese, A. C. (1980) in Photosensitization of
Organisms with Special Reference to Natural Photosensitizers
(Hillenkampf, F., Pratesi, R., and Sacchi, C., eds.) Plenum Press,
N. Y., pp. 299-319.
27.Zakharov, S. D. (1999) Kvant. Elektronika,
29, 192-214.
28.Karu, T. I. (1988) Lasers Life Sci.,
2, 53-64.
29.Vladimirov, Yu. A. (1994) in Efferent Medicine
(Chikin, S., ed.) Institute of Biomedical Chemistry, Russian
Academy of Medical Sciences, Moscow, pp. 51-66.
30.Romm, A. R., Sherstnev, M. P., Volkov, V. V., and
Vladimirov, Yu. A. (1986) Byul. Eksp. Biol. Med., 102,
426-428.
31.Gorbatenkova, E. A., Azizova, O. A., and
Vladimirov, Yu. A. (1988) Biofizika, 33, 717-718.
32.Vladimirov, Y. A., Gorbatenkova, E. A.,
Paramonov, N. V., and Azizova, O. A. (1988) Free Rad. Biol.
Med., 5, 281-286.
33.Vladimirov, Yu. A. (1965) Photochemistry and
Luminescence of Proteins [in Russian], Nauka, Moscow.
34.Vladimirov, Yu. A., Azizova, O. A., Deyev, A. I.,
Kozlov, A. V., Osipov, A. N., and Roschupkin, D. I. (1992) Free
Radicals in Living Systems, in Advances in Science and
Technology. Biophysics [in Russian], Vol. 29, VINITI, Moscow.
35.Osipov, A. N., Yakutova, E. Sh., and Vladimirov,
Yu. A. (1993) Biofizika, 38, 390-396.
36.Vladimirov, Yu. A., and Archakov, A. I. (1972)
Lipid Peroxidation in Biological Membranes [in Russian], Nauka,
Moscow.
37.Vladimirov, Y. A., Olenev, V. I., Suslova, T. B.,
and Cheremisina, Z. P. (1980) Adv. Lipid Res., 17,
173-249.
38.Vladimirov, Y. A. (1987) in Physicochemical
Aspects of Medicine. Soviet Medical Reviews (Lopukhin, Y. M., ed.)
Harwood Academic Publishers GmbH, London, pp. 51-127.
39.Hogg, N., Darley-Usmar, V. M., Wilson, M. T., and
Moncada, S. (1992) Biochem. J., 281 (Pt. 2), 419-424.
40.McCord, J. M. (1985) N. Engl. J. Med.,
312, 159-163.
41.McCord, J. M. (1986) J. Free Rad. Biol.
Med., 2, 307-310.
42.Cross, C. E., Halliwell, B., Borish, E. T.,
Pryor, W. A., Ames, B. N., Saul, R. L., McCord, J. M., and Harman, D.
(1987) Ann. Int. Med., 107, 526-545.
43.McCord, J. M. (1987) Fed. Proc.,
46, 2402-2406.
44.McCord, J. M. (1988) Free Rad. Biol. Med.,
4, 9-14.
45.Malinovskaya, S. L., Drugova, O. V., Monich, V.
A., and Mukhina, I. V. (1999) Byul. Eksp. Biol. Med.,
128, 302-304.
46.Drugova, O. V., Monich, V. A., and Zhitnikova, O.
V. (2001) Byul. Eksp. Biol. Med., 131, 325-326.
47.Klebanov, G. I., Chichuk, T. V., and Vladimirov,
Yu. A. (2001) Biol. Membr. (Moscow), 18, 42-50.
48.Rubtsov, B. V., Klebanov, G. I., Ruzhitsky, A.
O., and Vladimirov, Yu. A. (1984) Izv. AN SSSR, Ser. Biol.,
2, 299-302.
49.Rubtsov, B. V., Klebanov, G. I., Zamorin, O. V.,
and Vladimirov, Yu. A. (1984) Izv. AN SSSR, Ser. Biol.,
4, 623-626.
50.Klebanov, G. I., and Vladimirov, Yu. A. (1999)
Uspekhi Sovrem. Boil., 119, 461-474.
51.Morel, F., Doussiere, J., and Vignais, P. V.
(1991) Eur. J. Biochem., 201, 523-546.
52.Klebanov, G. I., Poltanov, E. A., and Vladimirov,
Yu. A. (2003) Biofizika, 48, 462-474.
53.Karu, T. I., Afanas'eva, N. I., Kolyakov, S. F.,
and Pyatibrat, L. V. (1998) Dokl. RAN, 360, 267-270.
54.Andreoni, A., Colasanti, A., Malatesta, V.,
Riccio, P., and Roberti, G. (1991) Photochem. Photobiol.,
53, 797-805.