REVIEW: Interaction of Pigment-Protein Complexes within Aggregates Stimulates Dissipation of Excess Energy
N. V. Karapetyan
Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, Moscow 119071, Russia; fax: (7-095) 954-2732; E-mail: nkarap@inbi.ras.ru
Received July 2, 2004
Pigment-protein complexes in photosynthetic membranes exist mainly as aggregates that are functionally active as monomers but more stable due to their ability to dissipate excess energy. Dissipation of energy in the photosystem I (PSI) trimers of cyanobacteria takes place with a contribution of the long-wavelength chlorophylls whose excited state is quenched by cation radical of P700 or P700 in its triplet state. If P700 in one of the monomer complexes within a PSI trimer is oxidized, energy migration from antenna of other monomer complexes to cation radical of P700 via peripherally localized long-wavelength chlorophylls results in energy dissipation, thus protecting PSI complex of cyanobacteria against photodestruction. It is suggested that dissipation of excess absorbed energy in aggregates of the light-harvesting complex LHCII of higher plants takes place with a contribution of peripherally located chlorophylls and carotenoids.
KEY WORDS: antenna chlorophyll, P700, energy dissipation, light-harvesting complex LHCII, photosystem I trimer
Abbreviations: C708 (C740, etc.)) chlorophylls with absorption maximum at 708 nm (740 nm, etc.); LHCII, LHCI) light-harvesting complexes of PSII and PSI, respectively; F760 (F730, etc.)) fluorescence band with emission maximum at 760 nm (730 nm, etc.); P700) primary electron donor of PSI; PSI, PSII) photosystems I and II, respectively.
Transformation of light energy into chemical energy in the course of
oxygenic photosynthesis of plants and cyanobacteria takes place with
the contribution of sequentially functioning photosystems II and I
(PSII, PSI), the membrane complexes containing reaction center and core
antenna. Chlorophylls in light-harvesting antenna complexes of PSII
(LHCII) or PSI (LHCI) of plants contribute in harvesting and migration
of absorbed energy to core antenna of both photosystems. The
relationship between light-harvesting and photosynthetic electron
transport regulates absorption of light energy and the efficiency of
its utilization in photosynthesis [1, 2].
The final efficiency of photosynthesis is determined not only by the activity of electron transport and CO2 fixation but also by the stability of the photosynthetic apparatus. Under conditions of intense illumination when not all of the energy absorbed by plants is utilized for CO2 fixation, the excess energy is dissipated into heat. In the course of evolution, photosynthetic organisms developed ability for photoprotection from the damaging action of light that is carried out in numerous mechanisms. Discharging of pigment antenna from the excess absorbed quanta takes place with contribution of non-photochemical quenching, while the discharging of electron transport chain takes place mainly due to the electron flow to different terminal oxidases [1-3].
Protection mechanisms of the photosynthetic apparatus of higher plants and algae are described in several reviews [1, 2, 4]. It is suggested that dissipation of the excess absorbed energy in plants takes place mainly via LHCII. The main component of non-photochemical quenching in plants is DeltapH-dependent quenching that triggers the violaxanthin cycle [4]. Less is known about protection mechanisms of the photosynthetic apparatus of cyanobacteria that contain no LHCII (and LHCI) and all chlorophyll is localized in PSI and PSII core antenna [5, 6]. Cyanobacteria are deficient in the violaxanthin cycle [7], which plays a significant role in the dissipation of excess energy in higher plants [4]. Phycobilisomes as the main antenna in cyanobacteria located out of thylakoids [8] transfer absorbed energy to PSII and PSI [9]. Recently it was shown that excess energy absorbed by phycobilisomes can be quenched by carotenes [10].
All these factors suggest the participation in protection of the photosynthetic apparatus of cyanobacteria mechanisms different from that in plants. In contrast to higher plants, cyanobacteria are highly enriched with PSI as compared with PSII: the PSI/PSII ratio is about unity in higher plants, but it is much higher in cyanobacteria, varying between 3-to-5.5 [9]. Thus, only part of PSI complexes in cyanobacteria can function in linear electron transport while another (main) part of PSI complexes are involved in PSI-driven cyclic electron transport. Another peculiarity of cyanobacteria is the efficient interaction of photosynthetic and respiratory electron transport chains that compensates insufficient electron flow from PSII to PSI [3, 11]. Such interaction was observed for higher plants also [12], but because of low rate of electron transport in the respiratory chain the contribution of respiratory electrons to the photosynthetic chain in plants is one order of magnitude lower than in cyanobacteria.
Exchange of excitation energy between the antennae of pigment-protein complexes might be one of the pathways of dissipation of excess energy. This problem has been under discussion for a long time because of dense packing of pigment-protein complexes in thylakoids. However it was shown that interaction of antennae of PSII in higher plant and the reaction center complexes of purple bacteria stimulates utilization of absorbed energy in charge separation that led to a gain in photochemistry [13]. Increase in the efficiency of utilization of absorbed energy was not observed in the course of interaction of PSI complexes [14, 15]. This review presents data on dissipation of excess absorbed energy as a result of interaction of antennae of monomer complexes within a PSI trimer of cyanobacteria and stimulation of energy dissipation in aggregates of LHCII trimers in higher plants. Organization of pigment antenna of PSI trimers of cyanobacteria and LHCII trimers of plants and the contribution of the long-wavelength chlorophylls and carotenoids in energy dissipation will be discussed.
STRUCTURE OF PSI COMPLEX OF CYANOBACTERIA
The PSI complex in the membrane of cyanobacteria is organized preferably as a trimer [6], while PSI complex in plants exists as a monomer bound with LHCI complexes [16, 17]. For long time the existence of PSI trimers in cyanobacteria was ascribed to an artifact of monomer aggregation as a result of detergent washing out in the course of their isolation. Direct evidence for the existence of PSI trimers in cyanobacterial thylakoids was obtained from the author's data showing that at 77 K the fluorescence spectra of cells of the filamentous cyanobacterium Spirulina (now Arthrospira) platensis and that of PSI trimers isolated from cells are characterized by an unusual emission band at 760 nm (F760) [18-20]. A long-wavelength emission band at 755 nm was observed also for another filamentous cyanobacterium, Pseudoanabaena sp. [21], but not for unicellular cyanobacteria Synechococcus (now Thermosynechococcus) elongatus and Synechocystis PCC6803 [22-25]. The existence of the PSI trimers in the membranes of the unicellular cyanobacteria was found using antibody [26] and electron microscopy [27]. The PSI trimer/monomer ratio in cyanobacterial membranes can be reversibly regulated: mainly trimeric complexes are isolated from membranes incubated with low salt, while preferably monomer complexes are isolated from membranes after incubation under high salt conditions [28, 29].
The molecular organization of PSI trimer of the cyanobacterium S. elongatus [30] and PSI monomer from bean [31] has been established. PSI complexes from prokaryotes and eukaryotes contain the same cofactors of the reaction center and electron transport and show similar energetics and kinetics of electron transport. Each monomer within a PSI trimer contains 12 polypeptides subunits and 127 cofactors including 96 chlorophyll molecules [30]. Polypeptides PsaL and PsaI are important in formation of PSI trimers in cyanobacteria [32]; mutants deficient in PsaL and PsaI subunits are unable to form trimers [33].
Tight binding of P700 with core antenna chlorophylls is a feature of PSI [34, 35], and therefore PSI reaction center complex cannot be isolated without antenna chlorophylls. The main part of the antenna chlorophylls in cyanobacteria is localized in PSI [9]. The high local concentration of antenna chlorophylls on PsaA/PsaB polypeptides of PSI complex should be noted. Antenna of the cyanobacterial PSI can be represented as a central part (10 chlorophyll molecules within membrane) and two peripheral layers on stromal (34 molecules) and lumenal (35 molecules) sides of the membrane [30, 36]. Almost all antenna chlorophylls are localized at a center-to-center distance of 7-16 Å that promotes fast excitation energy migration between chlorophylls.
Each monomer within a PSI trimer of cyanobacteria contains several beta-carotene molecules: 13-15 molecules in Synechocystis PCC6803 [37], 10-11 in S. platensis [38], 22 in S. elongatus [30]. All beta-carotenes are associated with PsaA/PsaB polypeptides within the membrane; no carotene is located close to P700. Carotene molecules are in close contact with 60 antenna chlorophylls (of 90) that should stimulate quenching of chlorophyll triplets [39]. Most of the beta-carotene molecules are localized near long-wavelength chlorophylls [30] and therefore they rather contribute in photoprotection. Carotenes may also play a structural role [40], participating in interaction of peripheral subunits with PsaA/PsaB, as well as in formation of PSI trimer [30, 41].
In addition to bulk chlorophylls, PSI of various cyanobacteria contains 3-10% of long-wavelength chlorophylls absorbing in the region of 700-750 nm [6, 38, 42-44]. The long-wavelength chlorophylls serve as terminal energy trap and therefore may significantly determine energy migration behavior in antenna and trapping by reaction center. Energy absorbed by those chlorophylls is efficient in P700 photooxidation [15, 45]; thus even small overlap between the emission band of long-wavelength chlorophylls and the absorption band of reaction center is enough for efficient energy transfer to reaction center. Long-wavelength chlorophylls increase absorption cross-section of far-red light under conditions of cyanobacterial growth in dim light [15, 46] or protect PSI complex against damage caused by absorbed excess energy when the reaction center is closed [6, 47, 48].
PSI antenna of cyanobacteria differs in content and spectral characteristics of long-wavelength (red) chlorophylls [6, 42, 43]. In contrast to PSI monomers, the PSI trimers of S. platensis contain the most red chlorophyll absorbing at 740 nm (C740) and emitting at 760 nm (F760) at 77 K [19, 29], while the PSI trimers and monomers of S. elongatus have the same spectral forms of red chlorophylls that differ only in chlorophyll content [45]. The existence of the long-wavelength chlorophylls of PSI trimers and monomers is visible as fluorescence emission even at room temperature [29] but most pronounced at low temperatures [29, 45, 49]. The main fluorescence emission band of PSI trimers and monomers of Synechocystis PCC6803 and S. elongatus at 77 K is peaked at 725-730 nm [22-25], whereas in PSI trimers of S. platensis another intense band peaking at 760 nm is observed in addition to 730 nm [19, 29]. The intensity of F760 of PSI trimers of S. platensis at 77 K depends strongly on P700 redox state: it is maximum with P700 reduced and it is about one order of magnitude lower with P700 oxidized [6, 19].
Red shift of absorption of the long-wavelength chlorophylls is ascribed to aggregates formed as a result of pigment-pigment interaction. Reconstruction of PSI trimers in the course of incubation of S. platensis PSI monomers within liposomes gives evidence that C740 is formed as a result of interaction of chlorophyll molecules localized on different monomer complexes. This confirms the peripheral location on PsaA/PsaB polypeptide of the longest wavelength chlorophyll [20]. The absorption band at 708 nm of PSI complexes of cyanobacteria is due to excitonic interaction of chlorophyll molecules since this band presents in circular dichroism (CD) spectra of PSI trimers and monomers [41, 50, 51]. The absence of the 740 nm band in the light induced CD spectrum may indicate that the reddest chlorophyll form is not in close proximity to P700 [41]. Various action of the electric field on absorption spectra of PSI complexes suggests different origin of red chlorophyll forms [52].
DISSIPATION OF EXCITATION ENERGY IN PSI OF CYANOBACTERIA
P700+ induced quenching. Long-wavelength chlorophylls contribute to dissipation of excess energy in PSI complex. In contrast to PSII and the photosystem of purple bacteria, PSI of plants and cyanobacteria show no intense variable fluorescence. This is an indication that P700 and P700+ are equal quenchers of fluorescence of antenna chlorophylls at 295 K [11]. Light induced accumulation of P700+ in PSI of cyanobacteria at 77 K leads to decrease rather than increase in PSI fluorescence [19, 29, 49]. Perhaps different mechanisms are involved in quenching of PSI fluorescence at room and low temperatures.
Energy absorbed by bulk chlorophylls migrates to P700 with almost 100% efficiency [53]. Elementary steps of energy transfer between neighboring antenna chlorophylls in PSI trimers of cyanobacteria take place in about 160 fsec, energy is equilibrated in bulk antenna for 360 fsec, and 3.6-9.8 psec in whole heterogeneous antenna [54]. Fast energy transfer from beta-carotene to chlorophyll in 105 fsec is due to high degree (85-90%) of energy migration. Kinetics of energy migration in PSI antenna of S. platensis was evaluated by fluorescence decay associated spectra (DAS) with sub-picosecond resolution. Red chlorophylls have no effect on energy equilibration in the heterogeneous antenna but delay trapping by reaction center: 11 psec in monomers and 15 psec in trimers of S. platensis [42]. The 20-50 psec lifetime components are ascribed to trapping of excitation energy by P700.
The fluorescence DAS of PSI complexes with picosecond [29] and femtosecond [42] resolution revealed no dependence of the kinetics of excitation energy migration on P700 redox state at 295 K. But such dependence was observed at 77 K in fluorescence DAS of PSI trimers of S. platensis [55, 56] and S. elongatus [49]. The energy migration steps between pools of different red chlorophylls have been resolved at 77 K in PSI trimers of S. platensis [55]. Decreased lifetime of some components (190 to 107 and 450 to 350 psec) was found in DAS of PSI trimers with P700+ as compared with that of P700. F760 of PSI trimers of S. platensis is quenched efficiently by P700+ since the value of the Foerster overlap integral between the F760 emission band and the P700+ absorption band is about two orders of magnitude higher in the case of P700+ as compared with that of P700 [55]. Although C740 in S. platensis is peripherally localized on PsaA/PsaB, a large distance from P700 (42 ?) is covered by high value of overlap integral that stimulates fast transfer of excitation energy from C740 to P700+ [56].
Triplet P700 induced quenching. When P700 is pre-oxidized, intense illumination of PSI trimers of S. elongatus leads to formation of triplets of antenna chlorophylls whose dissipation is accompanied by formation of carotenoid triplet [57]. If P700 and the PSI acceptor side cofactors (A1, FX, and FA/FB) in PSI trimers of S. platensis are pre-reduced, illumination causes charge separation and recombination of P700+ and A0- (primary electron acceptor of PSI reaction center in reduced state) results in the formation of P700 triplet with lifetime 1.1 msec at 5 K [58]. Similar kinetics of decay of P700 triplet and the quenching of F760 indicates that P700 triplet quenches the fluorescence of the reddest chlorophyll C740 [58, 59].
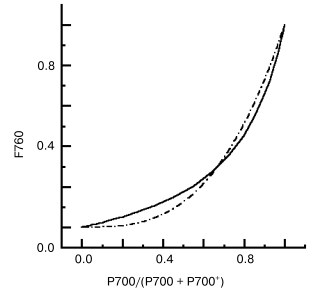
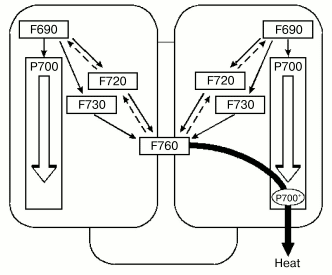
Quenching via interaction of pigment-protein complexes. Peripheral location of the reddest chlorophyll C740 in PSI trimers of S. platensis makes possible energy exchange between monomer antennas [6, 48]. Nonlinear dependence (Fig. 1) of the rate of F760 quenching on the relative content of P700, P700/(P700 + P700+), was observed by comparing the kinetics of P700 photooxidation and F760 quenching [48] and 77 K titration curves of accumulation of P700+ and the F760 quenching [59]. P700+ in PSI trimers of S. platensis is an efficient quencher of F760 [15, 56]. If P700 in one monomer complex within a PSI trimer is oxidized, excitation energy from antenna of monomer complexes with P700 reduced migrates via long-wavelength chlorophyll C740 (F760 on Fig. 2) to antenna of monomer complex with P700+ and is efficiently quenched. As follows from Fig. 1, 30% of P700+ (one P700 is oxidized in PSI trimer) quenches ~70% of F760, while 60% of P700+ (two P700 are oxidized in PSI trimer) quench about ~90% of F760. This energy exchange between monomer complexes stimulates non-radiative deactivation of the excess excitation energy, protecting the complex against destruction.
Fig. 1. Nonlinear dependence of F760 intensity on the relative content of P700red/P700 total in PSI trimers of the cyanobacterium Spirulina platensis, 77 K; continuous line, measured curve; dashed line, calculated curve [59].
Fig. 2. Scheme of energy exchange between the antennae of monomer complexes within PSI trimer of Spirulina platensis via long-wavelength chlorophylls and dissipation of energy into heat with contribution of P700+. Chlorophyll forms F690, F720, and F760 are indicated according to their 77 K fluorescence band [6].
DISSIPATION OF ENERGY IN LHCII AGGREGATES OF HIGHER PLANTS
LHCII complexes are widespread in thylakoids of higher plants and are the most abundant membrane complexes in nature. About 60% of all chlorophylls are bound with LHC proteins encoded by lhc (light harvesting complex) nuclear genes [16]. The LHCII pigments transfer the absorbed energy to PSII, thus increasing the optical absorption cross-section of the photoreaction [60]. LHCII complex is a trimer [16, 61, 62] consisting of 7-8 chlorophyll a, 5-6 chlorophyll b, 2 lutein, 1 neuxantin, and variable amount of violaxantin, zeaxanthin, and antheraxantin.
Comparison of the properties of LHCII trimers and monomers permits the understanding of the biological significance of the trimerization. Treatment of trimers by phospholipase results in no loss of chlorophylls of monomers but leads to spectral changes of carotenoids [63]. Trimerization effects the conformation of one of the lutein domains causing the quenching of LHCII fluorescence. The LHCII trimers are more stable compared with monomers. Orientation of pigments and protein conformation in trimers determine the efficient light harvesting and high protein stability as well as ability to control energy dissipation.
All LHCII complexes reveal dynamic behavior dependent of light intensity. The LHCII trimers strongly interact in vivo, i.e., they form an aggregate [64, 65]. It is suggested that LHCII trimers in chloroplasts form heptamers [66]. Aggregation of LHCII in vitro is accompanied by changes in structure [67] as well as by strong decrease in fluorescence yield. In contrast to LHCII trimers showing 77 K emission at 680 nm, LHCII aggregates show long-wavelength emission bands at 700, 695, and 685 nm emitted by the formed red chlorophylls [68]. The component qE of non-photochemical quenching that determines the physiologically important regulation mechanism of the PSII adaptability to excitation energy is related to formation of LHCII aggregate [69]. Allosteric binding of protons and zeaxanthin with LHCII protein causes conformational changes leading to quenching of excitation energy [70]. It was found that chlorophyll binding PsbS protein (22-kD protein of PSII), which is ascribed to the family of light harvesting proteins, contributes only to quenching but not to light harvesting [71-74]; the degree of fluorescence quenching in vivo correlates with content of PsbS [72].
The lower fluorescence yield of LHCII oligomers is indicative of an additional way of energy dissipation in oligomers. Investigation of the kinetics of fluorescence emission of isolated LHCII trimers and their oligomers shown that fluorescence lifetime of trimers is about 4.3 nsec, while that for trimer aggregates is much less (110 psec), thus indicating the efficient process of dissipation in aggregates [75]. Analysis of the fluorescence DAS in vitro shows that the long-wavelength chlorophylls are indirectly involved in fluorescence quenching of LHCII [68]. Carotenoids may control the oligomerization process of LHCII: zeaxanthin stimulates the formation of LHCIIb aggregates with lower fluorescence yield, while violaxanthin inhibits such aggregation [76].
Aggregation of LHCII complexes under excess light conditions leads to reversible structural changes that effect the energy migration and probably are involved in regulation of the pathways of energy dissipation in antenna [77]. Formation of chlorophyll triplet as well as fluorescence yield are increased by detergent induced disaggregation of LHCII complexes as a result of decrease in quenching of singlet excited state [78], although the efficiency of energy transfer from chlorophyll triplet to carotenes is slightly changed in course of disaggregation. No clear differences in inter- and intra-trimer energy transfer in LHCII were found [79]. It is suggested that energy transfer between chlorophyll molecules in LHCII is one order of magnitude slower than in PSI antenna because of lower density of chlorophyll molecules compared with PSI [61, 80].
Thus the interaction of pigment-protein complexes in PSI trimers of cyanobacteria and in aggregates of LHCII trimers leads to dissipation of excess absorbed energy increasing the stability of the photosynthetic apparatus under stress.
This study was supported by the Russian Academy of Sciences (grant within the Program “Molecular and Cellular Biology”) and Russian Foundation for Basic Research (grant 02-04-48348).
REFERENCES
1.Horton, P., Ruban, A. V., and Walters, R. G. (1996)
Annu. Rev. Plant Physiol. Plant Mol. Biol., 47,
655-684.
2.Niyogi, K. K. (1999) Annu. Rev. Plant Physiol.
Plant Mol. Biol., 50, 333-359.
3.Schmetterer, G. (1994) in The Molecular Biology
of Cyanobacteria (Bryant, D. A., ed.) Kluwer Academic Publishers,
Dordrecht, pp. 409-435.
4.Demmig-Adams, B., and Adams, W. W., III (1992)
Annu. Rev. Plant Physiol. Plant Mol. Biol., 43,
599-626.
5.Golbeck, J. H. (1994) in The Molecular Biology
of Cyanobacteria (Bryant, D. A., ed.) Kluwer Academic Publishers,
Dordrecht, pp. 319-360.
6.Karapetyan, N. V., Holzwarth, A. R., and Roegner,
M. (1999) FEBS Lett., 460, 395-400.
7.Hirschberg, J., and Chamovitz, D. (1994) in The
Molecular Biology of Cyanobacteria (Bryant, D. A., ed.) Kluwer
Academic Publishers, Dordrecht, pp. 559-579.
8.Gantt, E. (1994) in The Molecular Biology of
Cyanobacteria (Bryant, D. A., ed.) Kluwer Academic Publishers,
Dordrecht, pp. 119-138.
9.Rakhimberdieva, M. G., Boichenko, V. A.,
Karapetyan, N. V., and Stadnichuk, I. N. (2001) Biochemistry,
40, 15780-15788.
10.Rakhimberdieva, M. G., Stadnichuk, I. N.,
Elanskaya, I. V., and Karapetyan, N. V. (2004) FEBS Lett.,
574, 85-88.
11.Berry, S., Bolychevtseva, Y. V., Roegner, M., and
Karapetyan, N. V. (2003) Photosynth. Res., 78, 67-76.
12.Peltier, G., and Cournac, L. (2002) Annu. Rev.
Plant Biol., 53, 523-550.
13.Duysens, L. N. M. (1986) in Light Emission by
Plants and Bacteria (Govindjee, J., Amesz, D. C., and Fork, D. C.,
eds.) Academic Press, N. Y., pp. 4-32.
14.Karapetyan, N. V., Klimov, V. V., and Krasnovsky,
A. A. (1973) Photosynthetica, 7, 330-337.
15.Shubin, V. V., Bezsmertnaya, I. N., and
Karapetyan, N. V. (1995) J. Photochem. Photobiol., 30B,
153-160.
16.Jansson, S. (1994) Biochim. Biophys. Acta,
1184, 1-19.
17.Boekema, E. J., Jensen, P. E., Schlodder, E., van
Breemen, J. F. L., van Roon, H., Scheller, H. V., and Dekker, J. P.
(2001) Biochemistry, 40, 1029-1036.
18.Shubin, V. V., Murthy, S. D. S., Karapetyan, N.
V., and Mohanty, P. (1991) Biochim. Biophys. Acta, 1060,
28-36.
19.Shubin, V. V., Bezsmertnaya, I. N., and
Karapetyan, N. V. (1992) FEBS Lett., 309, 340-342.
20.Kruip, J., Karapetyan, N. V., Terekhova, I. V.,
and Roegner, M. (1999) J. Biol. Chem., 274,
18181-18188.
21.Duval, J. C., Thomas, J. C., and Choquet, Y.
(1986) Biochim. Biophys. Acta, 848, 352-358.
22.Wittmershaus, B. P., Wolf, V. M., and Vermaas, W.
F. J. (1992) Photosynth. Res., 31, 75-87.
23.Van der Lee, J., Bald, D., Kwa, S. L. S., van
Grondelle, R., Roegner, M., and Dekker, J. P. (1993) Photosynth.
Res., 35, 311-321.
24.Gobets, B., van Amerongen, H., Monshower, R.,
Kruip, J., Roegner, M., van Grondelle, R., and Dekker, J. P. (1994)
Biochim. Biophys. Acta, 1188, 75-85.
25.Palsson, L.-O., Dekker, J. P., Schlodder, E.,
Monshouwer, R., and van Grondelle, R. (1996) Photosynth. Res.,
48, 239-262.
26.Tsiotis, G., Haase, W., Mueller, S., Engel, A.,
and Michel, H. (1995) Eur. J. Biochem., 231, 823-830.
27.Westermann, M., Neuschaefer-Rube, O., Moerschel,
E., and Wehrmeyer, W. (1999) J. Plant Physiol., 155,
24-33.
28.Kruip, J., Bald, D., Boekema, E. J., and Roegner,
M. (1994) Photosynth. Res., 40, 279-286.
29.Karapetyan, N. V., Dorra, D., Schweitzer, G.,
Bezsmertnaya, I. N., and Holzwarth, A. R. (1997) Biochemistry,
36, 13830-13837.
30.Jordan, P., Fromme, P., Klukas, O., Witt, H. T.,
Saenger, W., and Krauss, N. (2001) Nature, 411,
909-917.
31.Ben-Shem, A., Frolov, F., and Nelson, N. (2003)
Nature, 426, 630-635.
32.Chitnis, V. P., and Chitnis, P. R. (1993) FEBS
Lett., 336, 330-334.
33.Jekow, P., Schubert, W.-D., Fromme, P., Kruip,
J., Chitnis, P. R., Roegner, M., and Saenger, W. (1996) Z.
Naturforsch., 51B, 195-199.
34.Karapetyan, N. V., Shubin, V. V., Rakhimberdieva,
M. G., Vashchenko, R. G., and Bolychevtseva, Y. V. (1984) FEBS
Lett., 173, 209-212.
35.Shubin, V. V., Karapetyan, N. V., and Krasnovsky,
A. A. (1986) Photosynth. Res., 9, 3-12.
36.Byrdin, M., Jordan, P., Krauss, N., Fromme, P.,
Stehlik, D., and Schlodder, E. (2002) Biophys. J., 83,
433-457.
37.Takahashi, Y., Hirota, K., and Katoh, S. (1985)
Photosynth. Res., 6, 183-192.
38.Karapetyan, N. V. (1998) Biol. Membr.
(Moscow), 15, 461-471.
39.Fromme, P., Jordan, P., and Krauss, N. (2001)
Biochim. Biophys. Acta, 1507, 5-31.
40.Moskalenko, A. A., and Karapetyan, N. V. (1996)
Z. Naturforsch., 51C, 763-771.
41.Shubin, V. V., Tsuprun, V. L., Bezsmertnaya, I.
N., and Karapetyan, N. V. (1993) FEBS Lett., 334,
79-82.
42.Gobets, B., van Stokkum, I. H. M., Roegner, M.,
Kruip, J., Schlodder, E., Karapetyan, N. V., Dekker, J. P., and van
Grondelle, R. (2001) Biophys. J., 81, 407-424.
43.Gobets, B., and van Grondelle, R. (2001)
Biochim. Biophys. Acta, 1507, 80-99.
44.Melkozernov, A. N. (2001) Photosynth.
Res., 70, 129-153.
45.Palsson, L.-O., Flemming, C., Gobets, B., van
Grondelle, R., Dekker, J. P., and Schlodder, E. (1998) Biophys.
J., 74, 2611-2622.
46.Trissl, H.-W. (1993) Photosynth. Res.,
35, 247-263.
47.Mukerji, I., and Sauer, K. (1989) in
Photosynthesis. Plant Biology, Vol. 8 (Briggs, W. R., ed.) Alan
R. Liss, N. Y., pp. 105-122.
48.Karapetyan, N. V., Shubin, V. V., and Strasser,
R. J. (1999) Photosynth. Res., 61, 291-301.
49.Byrdin, M., Rimke, I., Schlodder, E., Stehlik,
D., and Roelofs, T. A. (2000) Biophys. J., 9,
992-1007.
50.Cometta, A., Zucchelli, G., Karapetyan, N. V.,
Engelmann, E., Garlashi, F. M., and Jennings, R. C. (2000) Biophys.
J., 79, 3235-3243.
51.Engelmann, E., Tagliaube, T., Karapetyan, N. V.,
Garlaschi, F. M., Zucchelli, Z., and Jennings, R. C. (2001) FEBS
Lett., 499, 112-115.
52.Frese, R. N., Palacios, M. A., Azzizi, A., van
Stokkum, I. H. M., Kruip, J., Roegner, M., Karapetyan, N. V.,
Schlodder, E., van Grondelle, R., and Dekker, J. P. (2002) Biochim.
Biophys. Acta, 1554, 180-191.
53.Van Grondelle, R., Dekker, J. P., Gilbro, T., and
Sundstroem, V. (1994) Biochim. Biophys. Acta, 1187,
1-65.
54.Kennis, J. T. M., Gobets, B., van Stokkum, I. H.
M., Dekker, J. P., van Grondelle, R., and Fleming, G. R. (2001) J.
Phys. Chem., 105B, 4485-4494.
55.Holzwarth, A. R., Dorra, D., Mueller, M. G., and
Karapetyan, N. V. (1998) in Photosynthesis: Mechanisms and
Effects, Vol. 1 (Garab, G., ed.) Kluwer Academic Publishers,
Dordrecht, pp. 417-420.
56.Dorra, D., Fromme, P., Karapetyan, N. V., and
Holzwarth, A. R. (1998) in Photosynthesis: Mechanisms and
Effects, Vol. 1 (Garab, G., ed.) Kluwer Academic Publishers,
Dordrecht, pp. 587-590.
57.Schlodder, E., Paul, A., and Cetin, M. (2001) in
Proc. 12 Int. Photosynthesis Congr., CSIRO Publishing,
Melbourne, S6-015.
58.Witt, H., Bordignon, E., Carbonera, D., Dekker,
J. P., Karapetyan, N. V., Teutloff, C., Webber, A., Lubitz, W., and
Schlodder, E. (2003) J. Biol. Chem., 278,
46760-46771.
59.Schlodder, E., Cetin, M., Byrdin, M., Terekhova,
I. V., and Karapetyan, N. V. (2004) Biochim. Biophys. Acta, in
press.
60.Boichenko, V. A. (1998) Photosynth. Res.,
58, 163-174.
61.Kuehlbrandt, W., Wang, D. N., and Fujiyoshi, Y.
(1994) Nature, 367, 614-621.
62.Liu, Z., Yan, H., Wang, K., Zhang, J., Gui, L.,
An, X., and Chang, W. (2004) Nature, 428, 287-292.
63.Wentworth, M., Ruban, A. V., and Horton, P.
(2004) Biochemistry, 43, 501-509.
64.Bassi, R., and Dainese, P. (1992) Eur. J.
Biochem., 204, 317-326.
65.Garab, G., Kieleczawa, J., Sutherland, J. C.,
Bustamante, C., and Hind, C. (1991) Photochem. Photobiol.,
54, 273-281.
66.Dekker, J. P., van Roon, H., and Boekema, E. J.
(1999) FEBS Lett., 449, 211-214.
67.Ruban, A. V., Dekker, J. P., Horton, P., and van
Grondelle, R. (1995) Photochem. Photobiol., 61,
216-221.
68.Vasil'ev, S., Irrgang, K.-D., Schroetter, T.,
Bergmann, A., Eichter, H.-J., and Renger, G. (1997)
Biochemistry, 36, 7503-7512.
69.Horton, P., Ruban, A. V., Rees, D., Pascal, A.
A., Noctor, G., and Young, A. J. (1991) FEBS Lett., 292,
1-4.
70.Horton, P., Ruban, A. V., and Wentworth, M.
(2000) Phil. Trans. R. Soc. Lond. B., 355, 1361-1370.
71.Li, X.-P., Bjoerkman, O., Shih, C., Grossman, A.
R., Rosenquist, M., Jansson, S., and Niyogy, K. K. (2000)
Nature, 403, 391-395.
72.Li, X.-M., Gilmore, A. M., and Niyogi, K. K.
(2004) J. Biol. Chem., 277, 33590-33597.
73.Aspinall-O'Dea, M., Wentworth, M., Pascal, A.,
Robert, B., Ruban, A., and Horton, P. (2002) Proc. Natl. Acad. Sci.
USA, 99, 16331-163315.
74.Bergantino, E., Segalla, A., Brunetta, A.,
Teardo, E., Rigoni, F., Giacometti, G. M., and Szabo, I. (2003)
Proc. Natl. Acad. Sci. USA, 100, 15265-15270.
75.Mullineaux, C. W., Pascal, A. A., Horton, P., and
Holzwarth, A. R. (1993) Biochim. Biophys. Acta, 1141,
23-28.
76.Ruban, A. V., Phillip, D., Young, A. J., and
Horton, P. (1997) Biochemistry, 36, 7855-7859.
77.Barzda, V., Istokovics, A., Simidjiev, I., and
Garab, G. (1996) Biochemistry, 35, 8981-8985.
78.Barzda, V., Peterman, E. G., van Grondelle, R.,
and van Amerongen, H. (1998) Biochemistry, 37,
546-551.
79.Barzda, V., Gulbinas, V., Kananavicius, R.,
Cervinskas, V., van Amerongen, H., van Grondelle, R., and Valkunas, L.
(2001) Biophys. J., 80, 2409-2421.
80.Krauss, N., Schubert, W.-D., Klukas, O., Fromme,
P., Witt, H. T., and Saenger, W. (1996) Nature Struct. Biol.,
3, 965-973.