A New Alkalitolerant Yarrowia lipolytica Yeast Strain Is a Promising Model for Dissecting Properties and Regulation of Na+-Dependent Phosphate Transport Systems
R. A. Zvyagilskaya1* and B. L. Persson2
1Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, Moscow 119071, Russia; fax: (7-095) 954-2732; E-mail: renata_z@inbi.ras.ru2Department of Biochemistry and Biophysics, Stockholm University, S-106 91 Stockholm, and Department of Chemistry and Biomedical Sciences, Kalmar University, S-391 82 Kalmar, Sweden
* To whom correspondence should be addressed.
Received July 9, 2004
A newly isolated osmo-, salt-, and alkalitolerant Yarrowia lipolytica yeast strain is distinguished from other yeast species by its capacity to grow vigorously at alkaline pH values (9.7), which makes it a promising model organism for studying Na+-dependent phosphate transport systems in yeasts. Phosphate uptake by Y. lipolytica cells grown at pH 9.7 was mediated by several kinetically discrete Na+-dependent systems specifically activated by Na+. One of these, a low-affinity transporter, operated at high concentrations of extracellular phosphate. The other two, high-affinity systems, maximally active in phosphate-starved cells, were repressed or derepressed depending on the prevailing extracellular phosphate concentration and pH value. The contribution of Na+/Pi-cotransport systems to the total cellular phosphate uptake progressively increased with increasing pH, reaching its maximum at pH >= 9.
KEY WORDS: Yarrowia lipolytica, yeast, cytoplasmic membrane, phosphate transport, Na+/Pi-cotransport system, regulation
Abbreviations: CAPS) 3-[cyclohexylamino]-1-propanesulfonic acid; CCCP) carbonyl cyanide m-chlorophenylhydrazone; DiOC6(3)) 3,3´-dihexyloxacarbocyanine iodide; HPi) high phosphate medium; LPi) low phosphate medium; ER) endoplasmic reticulum.
Inorganic phosphate (Pi) is one of the major macronutrients
essential for all living organisms including yeasts. It is a structural
element of diverse cellular components, including nucleic acids,
proteins, phospholipids, and phosphosugars, a constituent of
energy-supplying reactions (glycolysis and oxidative phosphorylation),
and a regulator in signal transduction cascades. However, despite its
widespread occurrence, Pi is often present in very low
amounts in many ecosystems [1]. Therefore, yeasts,
like other organisms, have to evolve a sophisticated system for sensing
Pi availability and adjusting coordinated gene expression in
response to varying Pi levels. In Saccharomyces
cerevisiae, the PHO (phosphate-responsive signaling pathway)
regulatory pathway regulates expression of “PHO” genes
involved in sensing (scavenging), specific uptake, integrating, and
storing of Pi [2-7].
A primary step in the utilization of extracellular Pi is its uptake by plasma membrane transporters via a cotransport with H+ or Na+ [6]. Among all non-animal eukaryotic cells, most of the available information on Pi transport systems has been confined to the yeast Saccharomyces cerevisiae. In this fungus, three different systems have been proposed to be involved in the uptake of Pi from the cultivation medium. One of these, a so-called low-affinity system (with an apparent Km for extracellular Pi of approximately 1 mM at its pH optimum of 4.5), has been suggested to be a constitutively expressed H+/Pi cotransporter [8-10]. Recently, two new PHO genes, PHO90 and PHO91, have been cloned and sequenced. It was postulated that it is these genes that are encoding the low-affinity Pi-transporter [11].
Besides this low-affinity system, a growing family of high-affinity Pi transporters, potentially repressible by high Pi concentrations, is now believed to exist in S. cerevisiae [6, 7]. Of these, the most important is Pho84p, the product of the PHO84 gene [12], a 65-kD hydrophobic membrane protein, a H+/Pi-cotransporter, responsible for the major cellular Pi uptake at acidic or neutral pH values under Pi-limiting conditions. It is maximally active at pH 4.5 with an apparent Km value for Pi of 1-15 µM [13-16]. Pho84p belongs to the class of 12-transmembrane hydrophobic helix transporters, bearing a high degree of similarity to members of the yeast hexose transporter family [17, 18] and to both Snf3p and Rgt2p involved in sensing of external glucose concentrations [19]. Although Pho84p has been shown to be solely responsible for H+-coupled Pi uptake in model systems (plasma membrane vesicles enriched in Pho84p or proteoliposomes loaded with Pho84p) [16, 20-22], in vivo several other auxiliary proteins including Pho87p [23, 24], Pho88p [24], and Gtr1p [25], are proposed to be associated with the Pho84p-mediated transport system, possibly serving as receptors for Pi signaling, or altering the intrinsic stability of Pho84p, or affecting Pho84p activity in another, so far unknown manner. Under Pi starvation, the Pho84p is not only transcriptionally up-regulated, but being synthesized on ER (endoplasmic reticulum) cytoribosomes, is sorted to the final destination, i.e., the plasma membrane. The correct sorting of Pho84p depends on Pho86p, an ER resident protein, possibly required for proper packaging of Pho84p into COPII (coat protein complex II) vesicles, within them Pho84p is transported to the plasma membrane [26, 27]. In low-Pi-medium containing 250-300 µM Pi, the rate of Pho84p-mediated Pi uptake by S. cerevisiae gradually increases during exponential growth reaching its maximum at the late-exponential growth phase, and then rapidly declines [28]. The onset of the decline in Pi transporter activity coincides with decreasing of the extracellular Pi concentration to 10 µM, which is close to the reported Km value for the transporter [28]. These and other data support the idea that both the derepression and inactivation of Pho84p are under the control of extracellular phosphate level [12, 28]. As external phosphate is almost totally exhausted, the Pho84p is inactivated and routed to the vacuole to be degraded [5, 29].
The other high-affinity transporter of S. cerevisiae is the PHO89 gene product [30] catalyzing a Na+/Pi cotransport. The transporter is active predominantly at pH 9.5, with a Km for Pi of 1 µM at pH 7.2 [14, 30]. Like Pho84p, Pho89p is organized into 12 discrete hydrophobic domains [6] and displays a high degree of similarity with mammalian Na+/Pi transporters of type III. However, activity of the Na+-coupled Pho89p in S. cerevisiae is very low, casting some doubt on its physiological significance. Obviously, the S. cerevisiae yeast, thriving at pH 5.5-6.5 and only barely growing at pH >= 8.0, is not the best model organism for studying Na+-coupled transporters active predominantly under alkaline conditions. Clearly, more appropriate yeast species are needed to gain precise resolution of Pi transport mechanisms in yeast cells grown under alkaline conditions. For this purpose, in our studies on properties and regulation of Na+-dependent Pi-transport systems we used the recently isolated by us [31] osmo-, salt-, and alkalitolerant strain of the yeast Yarrowia lipolytica. The strain was isolated from salt-excreting leaves of desert plants, containing microorganisms well adapted to daily fluctuating and often extreme temperature, pH, and salinity [32]. Furthermore, the salt-excreting leaves of arid plants were commonly colonized by the novel Y. lipolytica strain, thus indicating its perfect adaptation to extreme growth conditions. The isolated strain shares all advantages of typical Y. lipolytica strains, being nontoxic, growing to very high densities, and having a haploid genome amenable to both classical and molecular genetic techniques. The new strain, however, differs from other typical Y. lipolytica strains and, more generally, from other yeast species, by its inherent ability to grow over a wide range of pH values, from 3.0 to 10.0 [31]. It is worthwhile to note that pH 10.0 is the upper pH limit for yeast growth. The overwhelming majority of yeast species thrives at pH 5.5-6.5 and can only barely grow at pH 8. The capacity to grow vigorously at alkaline pH values makes the new Y. lipolytica strain a promising model for clarifying general principles of yeast adaptation to extreme environmental factors and possibly an exceptionally useful tool in dissecting properties and regulation of Na+-coupled Pi-cotransport systems in yeasts.
Previously, we have shown that in Y. lipolytica cells grown at pH 4.5, Pi accumulation was mediated by two kinetically discrete H+/Pi-cotransport systems, maximally active at pH 5.5 with apparent Km values for Pi of 2-3 mM and 12-18 µM Pi, respectively [33, 34], closely matching the values reported for the Pi transporters in S. cerevisiae cells (see above). The low-affinity H+-coupled Pi-cotransport system operated at high Pi concentrations. The high-affinity H+/Pi-cotransport system came into play during Pi-starvation, being under the control of both extracellular Pi availability and intracellular polyphosphates stores [33, 34].
In this paper we report kinetic properties, regulation, and relative contribution to the total cellular Pi uptake of several kinetically distinct Na+-dependent Pi uptake systems of Y. lipolytica cells grown under alkaline conditions (pH 9.7).
MATERIALS AND METHODS
Chemicals. 3-[Cyclohexylamino]-1-propanesulfonic acid (CAPS), succinate, Tris, carbonyl cyanide m-chlorophenylhydrazone (CCCP), and glucose were purchased from Sigma (USA); Bacto Peptone, yeast extract, and Bacto Agar were from Difco (USA); KOH, KH2PO4, MgSO4, (NH4)2SO4, LiCl, KCl, and NaCl from Merck (Germany).
Organism and growth conditions. In this work we used the osmo-, salt-, and alkalitolerant strain of the Yarrowia lipolytica yeast obtained as a pure isolate from epiphytic microflora of salt excreting leaves of arid Atriplex halimus plant from the Negev Desert (Israel). On the basis of its morphological, physiological, biochemical, and chemotaxonomic characteristics and molecular-genetic analysis, the strain was identified as an anamorpha of Yarrowia lipolytica (Wick.) van der Walt and Arx or as a new variety, Y. lipolytica var. alkalitolerance [31].
Cells were routinely grown at 30°C on complex buffered agar-solidified medium containing either 1% yeast extract, 2% Bacto Peptone, 1% glucose, 0.2% KH2PO4, 2% agar (high phosphate medium, HPi) or 0.6% Bacto Peptone, 1% glucose, 2% agar, 0.05% MgSO4, 0.03% (NH4)2SO4, vitamins, microelements, approximately 300 µM Pi as traces from the reagents used (low phosphate medium, LPi). Culture media were autoclaved and then adjusted to the desired pH values with KOH and 2.5 M Tris-HCl-buffer (to the final concentration of 50 mM). Cultures grown on the buffered media were aseptically suspended in 50 mM Tris-HCl to 9 OD590 units/ml, and 200 µl of the cell suspensions were spread onto plates (1.8-1.9 OD590 units/plate) and allowed to grow for only 8-10 h at pH 7.0 or for 20 h at pH 9.7 to avoid considerable acidification during the growth. Cell growth was monitored at 590 nm (A590).
Phosphate uptake was assayed as described earlier [33-36] (see also figure legends). The transport process was initiated by addition to 30-µl cell suspension (0.546 mg dry weight) of 1 µl of 32P-labeled orthophosphate (0.18 Ci/µmol; 1 mCi = 37 MBq; Amersham-Pharmacia Biotech., Sweden). Incubation time was 20, 40, and 60 sec. Phosphate uptake was terminated by addition of 3 ml of ice-cold dilution buffers. The cell suspensions were immediately filtered, the Whatman GF/F filters (Whatman, UK) were washed once with the same cold dilution buffers, and the radioactivity retained on the filters was determined by liquid scintillation spectrometry. To assay activity of the H+/Pi-cotransport system, yeast cells were washed by 25 mM Tris-succinate buffer, pH 5.5, and suspended in the same buffer, supplemented with 0.11 mM Pi and 3% glucose. To assay activity of the Na+/Pi-cotransport system, cells were washed by 25 mM CAPS-Tris-buffer, pH 9.5, and suspended in the same buffer, supplemented with 0.11 mM Pi, 3% glucose, and 20 mM NaCl. The initial rate of Pi uptake was estimated for the first minute. Every point on figures is the average from four to five determinations using the same cell preparation ± SE; experiments were run at least 4 times.
RESULTS AND DISCUSSION
First, we elaborated a procedure allowing to rigorously maintain the desired pH values of buffered media in order to attain maximal activities of both H+- and Na+-coupled Pi transport systems. We empirically optimized the composition of LPi-medium, the number of cells used as inoculum, and the time period for cell growth.
Y. lipolytica cells grown in LPi medium at pH 9.7, in spite of these severe conditions, preserved full viability (as judged from the propidium iodide test) and generated high transmembrane potential (Deltaiota) (monitored with fluorescent probes by flow cytometry) in both the mitochondrial and plasma membrane compartments [34]. The Deltaiota-fluorescence related to the mitochondrial potential (measured with 2 nM DiO6(3)) was sensitive to the uncoupler CCCP (50 µM), which is in harmony with direct measurements of oxidative and phosphorylative activities of high-quality mitochondria isolated from Y. lipolytica cells grown at pH 9.7 [34]. In contrast, the plasma membrane potential (visualized with 20 nM DiO6(3)) was not significantly affected even in the presence of 140 µM CCCP [33], suggesting that the observed hyperpolarization of the plasma membrane was not predominantly due to the H+ electrochemical gradient.
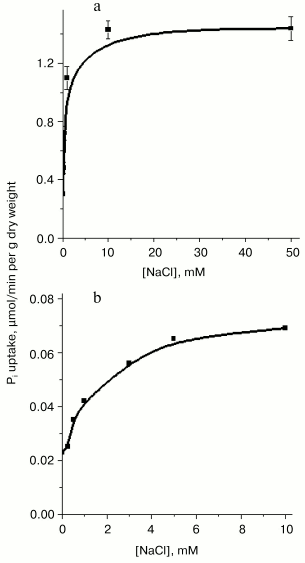
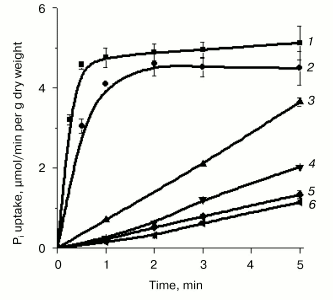
In contrast to Y. lipolytica cells grown at pH 4.5, where Pi accumulation was independent of Na+ concentrations in the incubation medium [33, 34], cells grown at pH 9.7 in LPi- and HPi-media containing the lowest attainable Na+ concentration as trace amounts from the reagents used, exhibited an almost negligible Pi uptake (Figs. 1 and 2). The initial rate of Pi uptake was strongly promoted by increasing external Na+ concentrations (up to 10 mM), especially in cells grown on LPi-medium (Figs. 1a and 2). The stimulatory effect of Na+ was highly specific; other ions of alkali metals (K+ or Li+) did not potentiate Pi uptake (Fig. 2, curves 5 and 6). Pi uptake activity was dependent on the presence of glucose as an energy substrate (Fig. 2, curve 3) and was practically insensitive to the uncoupler CCCP (Fig. 2, curve 2). These results suggest that Pi uptake by Y. lipolytica cells grown under alkaline conditions is mediated by transport systems differing from those engaged in Pi accumulation in Y. lipolytica cells grown at acidic conditions and that this transport was most likely driven by a symport with Na+. In the numerous well-documented cases of the Na+-substrate symport, found mainly in animal cells and alkalophilic bacteria, Na+ extrusion pumps transfer Na+ out of the cell against its electrochemical potential gradient, thus establishing a transmembrane DeltaµNa+. The downhill return flux of Na+ ions is coupled to inward flux of the symported substrate, in this case Pi.
Fig. 1. Stimulatory effect of NaCl on Pi uptake by Y. lipolytica cells grown at pH 9.7 in LPi (a) and HPi (b) medium. Cells grown as described in “Materials and Methods” were washed twice in 25 mM CAPS-Tris, pH 9.5, and suspended in the assay buffer containing 25 mM CAPS-Tris, pH 9.5, 0.11 mM Pi, and 3% glucose in the presence of various NaCl concentrations.
Pi transport in Y. lipolytica cells grown in both HPi- and LPi-media at pH 9.7 was similarly dependent on the external pH. Activity was maximal at pH 5.5-9.5, decreasing to one-third at pH 3.5 and diminishing to almost zero at pH 11 [34, 35]. The rather high transport activity seen at pH 6.5-8.5 was presumably due to fast expression of the H+/Pi-cotransport system under appropriate assay conditions. It is worthwhile to note that in Y. lipolytica grown in LPi-medium at pH 9.7, the initial net uptake of Pi measured during the first 15 and 30 sec under optimal conditions (the assay buffer contained 25 mM CAPS-Tris, pH 9.5, 20 mM NaCl, 0.11 mM Pi, and 3% glucose) corresponded to internalization of approximately 55 and 80% of the total added radioactivity, respectively (Fig. 2), which was essentially more than that reported for S. cerevisiae [30].Fig. 2. Effect of alkali ions and CCCP on Pi uptake by Y. lipolytica cells grown in LPi-medium at pH 9.7. Cells were washed twice in 25 mM CAPS-Tris, pH 9.5, and incubated in 25 mM CAPS-Tris, pH 9.5, containing 0.11 mM Pi and 3% glucose in the absence of any alkali ion (4) or in the presence of 25 mM Na+ (1), 25 mM K+ (5), 25 mM Li+ (6), or 60 µM CCCP + 25 mM NaCl (2). In the control experiment, the assay buffer was supplemented with 25 mM NaCl but glucose was omitted (3).
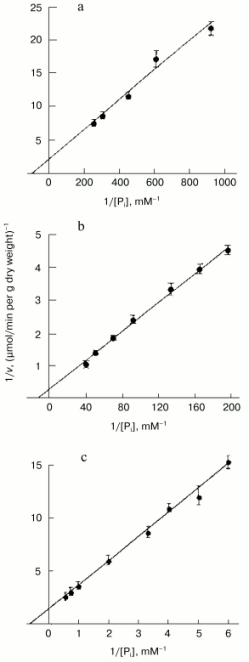
The high level of Pi internalization observed under optimal conditions in Pi-starved Y. lipolytica cells (Figs. 1a and 2), but lacking in HPi-grown cells (Fig. 1b), suggested the presence of at least two different Na+-dependent carrier systems responsible for the active Pi uptake under alkaline conditions. To investigate this possibility, we assessed the kinetic properties of Pi transport systems expressed in HPi- and LPi-grown cells by measuring uptake activity over a wide Pi concentration range (1 µM to 2 mM). The uptake reaction measured in cells grown in HPi-medium at pH 9.7 in the presence of 20 mM Na+ and various concentrations of Pi (0.167 to 2 mM) could be described by Michaelis-Menten kinetics with an apparent Km for Pi of 0.6 mM (Fig. 3c). Because at pH 9.7 less than 5% of the Pi is in the form of the monovalent anion [36], the apparent Km derived from Fig. 3 has to be corrected by a factor of 20 or more. The Pi accumulation by cells grown in LPi-medium at pH 9.7 was also measured in the presence of 20 mM Na+ and increasing Pi concentrations from 1.1 to 25 µM. Lineweaver-Burk plots describing Pi uptake as a function of external Pi concentration typically revealed two linear phases (Fig. 3, a and b), suggesting a multiphasic mechanism of Pi uptake with different affinities of the transporter for substrate(s). The term “multiphasic” in this context refers only to the shape of the reciprocal graphs, regardless of the mechanism of the uptake. Lineweaver-Burk plots for Pi concentrations ranging from 1.1 to 5.2 µM (Fig. 3a) and from 5.2 to 25 µM (Fig. 3b) indicated Km values for Pi uptake of 10.7 and 87 µM, respectively, the values that reflect an overestimation of at least 20-fold with respect to monovalent phosphate anion. Thus, Y. lipolytica cells grown under alkaline conditions exhibited both low- and high-affinity Na+/Pi-cotransport systems. The dramatic difference in the Pi uptake rate observed in Y. lipolytica cells grown in LPi- and HPi-media at pH 9.7 (Figs. 1, a and b) indicates that the Na+/Pi-cotransport systems, like the H+/Pi-cotransport systems of these cells [33, 34], are under the control of the availability of the extracellular Pi concentration.
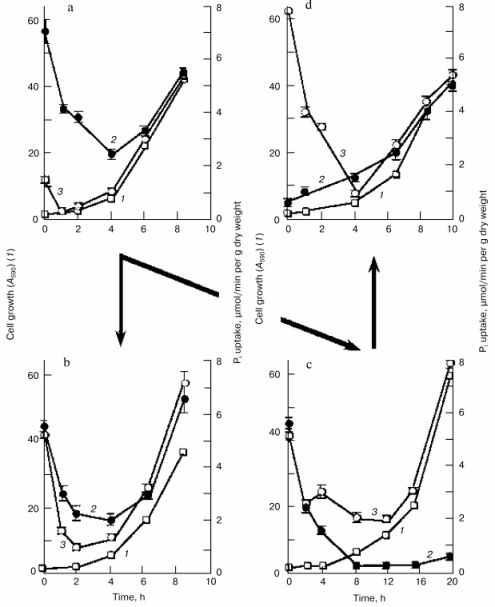
To get further insight into the regulation of the high-affinity H+/Pi- and Na+/Pi-cotransport systems, we measured Pi uptake by Y. lipolytica cells following their growth on LPi-medium at different pH values. To assess activity of the H+/Pi-cotransport system, cells were incubated in 25 mM Tris-succinate buffer, pH 5.5, supplemented with 0.11 mM Pi and 3% glucose [33, 34], while the activity assayed in 25 mM CAPS-Tris, pH 9.5, 20 mM NaCl, 0.11 mM Pi, and 3% glucose was taken as a measure of the Na+/Pi-cotransport system. Cells grown on LPi-medium at pH 7.0 (Fig. 4a) were used as a starting material to initiate growth of Y. lipolytica cells on LPi-media at pH 7.0 (as a positive control; Fig. 4b) and pH 9.7 (Fig. 4c). In both cases, cell transfer to the fresh portion of LPi-media was accompanied by an initial transient drop in activity of both H+/Pi- and Na+/Pi-high-affinity cotransport systems, possibly as a result of their repression by relatively high (300 µM) extracellular phosphate concentrations, followed by their recovery in a clearly pH-dependent manner. At pH 7.0, activities of both high-affinity H+/Pi- and Na+/Pi-cotransport systems were almost synchronously restored to their original high levels (Fig. 4b), while at pH 9.7, only the Na+-coupled Pi transport system was re-established (Fig. 4c), attaining very high (record) activity (7.8 µmol Pi consumed per min per g dry weight). When cells grown at pH 9.7 (Fig. 4c) were transferred to LPi-medium, pH 7.0 (Fig. 4d), activity of the Na+/Pi-cotransport system initially declined and then recovered with a concomitant enhancement of the H+/Pi-cotransport system, with a ratio of the H+/Na+-coupled Pi transport systems close to unity. Remarkably, the dramatic changes in activities of both H+- and Na+-coupled high-affinity Pi transport systems upon cell transfer to fresh portions of LPi-medium occurred when cell growth was essentially absent; therefore, they could not be explained by the dilution effect during cell growth. Rather, in the presence of repressible Pi concentrations, these transport systems may be inactivated or rapidly degraded, as it was demonstrated for the high-affinity H+/Pi-cotransport system of S. cerevisiae [37]. No growth was seen for Y. lipolytica cells precultured at pH 9.7 and then transferred to LPi-medium, pH 4.5. Moreover, this transfer was accompanied by cell lysis concurrent with an increase in extracellular Pi concentration. The rate of Pi accumulation by Y. lipolytica cells grown on HPi-medium at different pH values was very low (~0.3 µmol Pi consumed per min per g dry weight) and largely independent of the prevailing growth phase.Fig. 3. Lineweaver-Burk plots describing Pi uptake by Y. lipolytica cells grown in LPi- (a, b) and HPi- (c) media at pH 9.7 as a function of external Pi concentration. Cells were incubated in 25 mM CAPS-Tris, pH 9.5, containing 3% glucose, 20 mM NaCl, and Pi ranging from 1.1 to 5.2 µM (a), from 5.2 to 25 µM (b), and from 0.167 to 2 mM (c).
Thus, several novel kinetically discrete Na+-dependent Pi transport systems in Y. lipolytica cells grown under alkaline conditions have been revealed and characterized. One of these, a low-affinity Na+-dependent transporter with a Km value of 0.6 mM for monophosphate is most likely constitutively expressed at high extracellular Pi concentrations. The other two Na+-dependent high-capacity, high-affinity systems underwent repression-derepression depending on the prevailing extracellular Pi concentration and pH value. The quantification of the relative contribution of the H+- and Na+-coupled Pi transport systems to the total Pi uptake cellular activity clearly showed that the H+/Pi transport systems driven by the H+-gradient generated across the plasma membrane as a result of H+-ATPase operation, provided most, if not all, of the Pi uptake into Y. lipolytica cells grown at pH 4.5 and 6.0 [34, 38]. Previously, we have shown [34] that the cytoplasmic membrane of Y. lipolytica cells contains highly active H+-ATPase. Unfortunately, these data are the only available on ATPases of Y. lipolytica cells. The contribution of the Na+/Pi cotransport systems into the total cellular Pi uptake activity increased with increasing pH values, reaching its maximum at pH >=_9, where Pi accumulation was preferentially, if not exclusively, maintained through the Na+/Pi cotransport systems (Fig. 4c). At pH 7.0, both H+/Pi and Na+/Pi cotransport systems were equally responsible for Pi uptake (Fig. 4, a, b, and d).Fig. 4. Time course of 32P-labeled orthophosphate accumulation by Y. lipolytica cells during growth in LPi-medium at pH 7.0 (a, b, d) and 9.7 (c). Cells grown at pH 7.0 (a) were aseptically collected by centrifugation at 2300g for 15 min and divided into two portions, one of which was washed with 25 mM Tris-HCl buffer, pH 7.0, while the other with 25 mM Tris-HCl buffer, pH 9.5. These cell portions were used as starting materials for growth on LPi-media, pH 7.0 (b) or pH 9.7 (c), respectively. Similarly, cells grown at pH 9.7 (c) were transferred to LPi-medium, pH 7.0 (d). Cells were aseptically collected and A590 (1) and activities of the H+/Pi- (2) and Na+/Pi-cotransport systems (3) were determined.
In summary, the findings presented in this paper show the advantage of Y. lipolytica as a model system in studying of Pi transport upon large fluctuations in pH values of the growth medium and especially at alkaline pH values. To our knowledge, the yeast low-affinity Na+-dependent Pi uptake system was kinetically characterized for the first time. We also pioneered in showing that yeasts are endowed with a high-capacity, high-affinity, finely controlled Na+-dependent Pi acquisition system. This required the appropriate yeast species growing at alkaline pH values and optimization of growth conditions in order to rigorously maintain the desired pH values during cell growth. It was shown for the first time that both H+/Pi- and Na+/Pi-cotransport systems are under dual control by the prevailing extracellular Pi concentration and pH value. Altogether, these results contribute to the general knowledge of strategies underlying adaptation of Y. lipolytica yeast to varying growth conditions.
This study was supported by the Russian Academy of Sciences (grant on cellular and molecular biology), by the Russian Foundation for Basic Research (grant 03-04-48388), and by the Royal Swedish Academy of Sciences.
REFERENCES
1.Harold, F. M. (1966) Bacteriol. Rev.,
30, 772-794.
2.Lenburg, M. E., and O'Shea, E. K (1996) Trends
Biochem. Sci., 21, 383-387.
3.Oshima, Y. (1997) Gen. Genet. Syst.,
72, 323-334.
4.Persson, B. L., Petersson, J., Fristedt, U.,
Weinander R., Behre, A., and Pattison-Granberg, J. (1999) Biochim.
Biophys. Acta, 142, 255-272.
5.Lagerstedt, J. O., Kruckenberg, A. L., Berden, J.
A., and Persson, B. L. (2000) in Molecular Biology and Physiology of
Water and Solute Transport: Fundamental Research and Applied
Aspects (Hohmann, S., and Nielsen, S., eds.) Kluwer/Plenum, New
York, pp. 405-414.
6.Persson, B. L., Lagerstedt, J. O., Pratt, J. R.,
Pattison-Granberg, J., Kent, L., Shokrollahzaden, S., and Kent, F.
(2003) Curr. Genet., 43, 225-244.
7.Giots, F., Donaton, M. C. V., and Thevelein, J. M.
(2003) Mol. Microbiol., 47, 1163-1181.
8.Borst-Pauwels, G. W. F. H. (1981) Biochim.
Biophys. Acta, 650, 88-127.
9.Nieuwenhuis, B. J. W. M., and Borst-Pauwels, G. W.
F. H. (1984) Biochim. Biophys. Acta, 770, 40-46.
10.Tamai, Y., Toh-e, Y., and Oshima, Y. (1985) J.
Bacteriol., 164, 964-968.
11.Wykoff, D. D., and O'Shea, E. K. (2001)
Genetics, 159, 1491-1499.
12.Bun-ya, M., Nishimura, N., Harashima, S., and
Oshima, Y. (1991) Mol. Cell. Biol., 11, 3229-3238.
13.Cockburn, M., Earnshaw, P., and Eddy, A. A.
(1975) Biochem. J., 46, 705-712.
14.Roomans, G. M., and Borst-Pauwels, G. W. F. H.
(1979) Biochem. J., 178, 521-527.
15.Borst-Pauwels, G. W. F. H. (1993) Biochim.
Biophys. Acta, 1152, 201-206.
16.Behre, A., Fristedt, U., and Persson, B. L.
(1995) Eur. J. Biochem., 227, 566-572.
17.Bisson, L. F., Coons, D. M., Kruckenberg, A. L.,
and Lewis, D. A. (1993) Crit. Rev. Biochem. Mol. Biol.,
28, 259-308.
18.Henderson, P. J. F. (1993) Curr. Opin. Cell.
Biol., 5, 708-721.
19.Ozcan, S., Dover, J., Rosenwald, A. G., Wolfi,
S., and Jonston, M. (1996) Proc. Natl. Acad. Sci. USA,
93, 12428-12432.
20.Berhre, A., Norling, B., and Persson, B. L.
(1996) Arch. Biochem. Biophys., 330, 133-141.
21.Fristedt, U., Weinander, R., Martinsson, H. S.,
and Persson, B. L. (1999) FEBS Lett., 458, 1-5.
22.Fristedt, U., van Der Rest, M., Poolman, B.,
Konings, W. N., and Persson, B. L. (1999) Biochemistry,
38, 16010-16015.
23.Bun-ya, M., Shikata, K., Nakade, S., Yompakdee,
C., Harashima, S., and Oshima, Y. (1996) Curr. Genet.,
29, 344-351.
24.Yompakdee, C., Ogawa, N., Harashima, S., and
Oshima, Y. (1996) Mol. Gen. Genet., 251, 580-590.
25.Bun-ya, M., Harashima, S., and Oshima, Y. (1992)
Mol. Cell. Biol., 12, 2958-2966.
26.Lau, W. T., Howson, R. W., Malkus, P., Schekman,
R., and O'Shea, E. K. (2000) Proc. Natl. Acad. Sci. USA,
97, 1107-1112.
27.Yompakdee, C., Bun-ya, M., Shikata, K., Ogawa,
N., Harashima, S., and Oshima, Y. (1996) Gene, 171,
41-47.
28.Martinez, P., Zvyagilskaya, R., Allard, P., and
Persson, B. L. (1998) J. Bacteriol., 180, 2253-2256.
29.Petersson, J., Pattison, J., Kruckenberg, A. L.,
and Persson, B. L. (1999) FEBS Lett., 462, 37-42.
30.Martinez, P., and Persson, B. L. (1998) Mol.
Gen. Genet., 258, 628-638.
31.Zvyagilskaya, R., Andreishcheva, E., Soares, M.
I. M., Khozin, I., Behre, A., and Persson, B. L. (2001) J. Basic
Microbiol., 41, 289-303.
32.Simon, R., Abeliovich, A. V., and Belkin, S.
(1994) FEMS Microbiol. Ecol., 14, 99-110.
33.Zvyagilskaya, R., Allard, P., and Persson, B. L.
(2000) IUBMB Life, 49, 143-147.
34.Zvyagilskaya, R., Parchomenko, O., Abramova, N.,
Allard, P., Panaretakis, T., Pattison-Granberg, J., and Persson, B. L.
(2001) J. Membr. Biol., 183, 39-50.
35.Zvyagilskaya, R., Parchomenko, O., Abramova, N.,
and Persson, B. L. (2000) IUBMB Life, 50, 151-155.
36.Vinogradov, A. D., and Leikin, Y. N. (1971)
Biokhimiya, 36, 1061-1064.
37.Lagerstedt, J. O., Zvyagilskaya, R., Pratt, J.,
Pattison-Granberg, J., Kruckenberg, A. L., Berden, J. A., and Persson,
B. L. (2002) FEBS Lett., 526, 31-37.
38.Zvyagilskaya, R., and Persson, B. L. (2003)
IUBMB Life, 55, 151-154.