Bacteriorhodopsin. Correspondence of the Photocycle and Electrogenesis with Sites of the Molecule
L. V. Khitrina* and A. L. Ksenofontov
Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119992 Moscow, Russia; fax: (7-095) 939-3181; E-mail: khitr@phtbio.genebee.msu.su; ksenofon@belozersky.msu.ru* To whom correspondence should be addressed.
Received July 21, 2004
Correspondence of phases of electrogenesis, photocycle transitions, and proton transfer with the proton transporting groups of bacteriorhodopsin was studied. The structure of bacteriorhodopsin was considered by the file 1c3w and projections of sites of the proton movement pathway onto the normal to the purple membrane were measured. The dielectric permeability of the terminal site of the semichannel Schiff base --> external surface of the purple membrane was noticeably higher than in the center of the membrane.
KEY WORDS: bacteriorhodopsin, purple membranes, 1c3w, electrogenesis, proton transport, photocycle, distance between proton-transporting groups
Abbreviations: BR) bacteriorhodopsin; PM) purple membrane; SB) Schiff base.
Bacteriorhodopsin (BR) is a bacterial photo-dependent proton pump [1]. Absorption of a light quantum results in a cycle
of spectral transformation in the molecule of BR. The photocycle
transitions correspond to conformational changes in the molecule, which
provide the charge transfer across the membrane. A single turnover of
the photocycle results in transfer of not a single proton but of a
number of protons between certain groups, and this ensures the transfer
of one H+ across the whole thickness of the purple membrane
(PM) [2]. Although the fundamental construction of
the proton transport chain is more or less clear [2-5], functions of its separate
sites need to be detailed. The hypotheses describing the functions of
every proton-transporting site should reasonably agree with the
experimentally observed potential difference generated at the stage
under study and the distance traveled by the charges perpendicularly to
the PM surface. In our previous paper [2],
attention was paid to this agreement, and the purpose of the present
work was to study this problem more in detail.
MATERIALS AND METHODS
To obtain the electric response, the following approaches were used.
1. PMs were isolated from Halobacterium salinarum (halobium) culture by a usual method [1, 6, 7].
2. PMs were associated with an artificial flat lecithin membrane reinforced with a collodion film [2, 7].
3. The potential difference in the resulting system were directly determined [2, 7]. The electrogenesis was induced by a laser light flash, which caused a single turnover of the photocycle.
RESULTS AND DISCUSSION
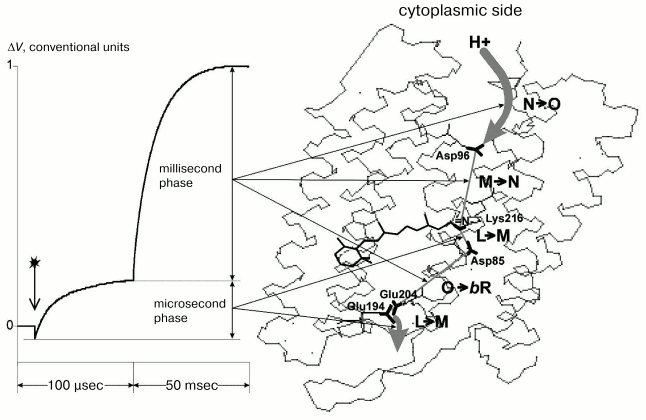
The figure presents a typical electric response of BR. The potential difference on the conjugating membrane is caused by the transfer across it of a charged particle--H+. The photopotential (DeltaV) at every stage of the process is directly proportional to the projection (r) of the pathway of the proton movement onto the normal to the membrane and inversely proportional to the dielectric constant (epsilon) of the membrane: DeltaV ~ kr/epsilon. Different phases of this response with the photocycle intermediates and structure of the molecule have been compared in detail earlier [2, 3], and this comparison is summarized in the figure with regard to new literature data [5, 8, 9] and our findings. The file with 1c3w coordinates [10] contains the best current X-ray diffraction analysis data on the BR molecule and is widely used in modern studies on BR. To evaluate the distances, we have also used the 1c3w structure of BR [10] with resolution of 1.55 Å. Moreover, according to the earlier comparison [2], sufficiently close values can be also obtained for other similar structures.
The 1c3w structure is not explicitly oriented with respect to PM. Therefore, we used the file 1BRR [11] with the BR trimer. Due to symmetric positions of amino acid residues in three BR molecules, it is easy to draw a plane through the same amino acid residues parallel to the sought-for membrane surface. The perpendicular to this plane is just the interesting for us direction of the normal to the membrane [9]. To measure projections of the distances onto the normal to the membrane, we have oriented the 1c3w structure using the trimer 1BRR (table).Correspondence of the electrogenesis phases, photocycle transitions, and proton transfer with groups of the proton transport chain of BR. To the left the typical electric signal of the light-adapted BR at room temperature is shown. The PMs are associated with the collodion film impregnated with lecithin solution in decane (70 mg/ml). The moment of the laser flash (lambda = 532 nm, t1/2 = 15 nsec, energy in the green light is 50 mJ) is shown by the arrow. Incubation medium: 100 mM NaCl, 5 mM Mes, 3 mM citrate, pH 6.0. To the right the structure of the file 1c3w obtained with the Skeleton program of Ya. L. Kalaidzidis is visualized
Distances (R) and their projections (r) onto the normal to PM in
the BR structure (taken from the file 1c3w [10],
see text)
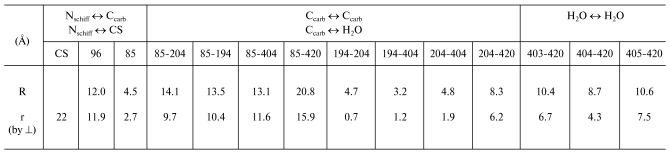
Notes: CS) cytoplasmic surface, its position in the file is
determined as in [9]. The numbers 403, 404, 405,
and 420 are designations of water molecules according to the author's
numeration of the file 1c3w. Although H+ is transmitted with
involvement of oxygen and not of carbon of the carboxyl group, it is
not clear which oxygen (or both) is involved in the transport chain,
and the difference in the distances measured from the oxygen or carbon
is less than 1 Å (see Fig. 3 from work [2]) at the accuracy of 1.55 Å determined by
X-ray diffraction analysis, we have taken everywhere the distances to
the carbon of the beta- or gamma-carboxyl groups of Asp
or Glu residues, respectively.
It is reasonable to think [9] that Lys40, 41, 159 and Asp36, 38, 102, 104 are located near the polar heads of the PM lipids on the surface which separates the hydrophobic and water-exposed regions of BR. The plane going through three Lys41 residues in different molecules of trimer 1BRR (NZ-atoms of each lysine) may be to a first approximation considered to be the surface of the membrane, neglecting probable hydrophilic pockets (for details see [9]).
The fast phase of the electric response corresponding to generation of the M-intermediate is associated with transfer of the H+ in two sites of the channel SB --> the periplasmic surface of the PM, and amplitudes of these contributions are approximately equal [1, 2, 12]. The first one is nearly in the middle of the membrane between the SB and Asp85. The projection of the distance between the nitrogen of the SB and carbon of the protonating beta-carboxyl group of Asp85 onto the normal to the membrane is 2.7 Å. For the other site of the channel between Glu204 and Glu194 this value is 0.7 Å. These data contradict the hypothesis about equal contributions of these sites to the generation of DeltaV, because lengths of the projections are nearly fourfold different, and this is difficult to be compensated by differences in the dielectric permeability. At least, it would be reasonable to expect the lower value of epsilon in the center of the membrane rather than the reverse. Therefore, the second site cannot be located between these residues. It has been shown earlier that neither Glu204 nor Glu194 are terminal groups of the proton transport chain, although carboxyl groups of both residues are involved in electrogenesis [2, 8, 12, 13]. We shall not discuss hypotheses concerning in detail the proton transmission through these carboxyls (whether the proton located on one of them or a water molecule between them (Nos. 403, 404, 405) is involved in the transport chain). In any case, the proton reaches the external (periplasmic) surface of the membrane. The file sites available for water were calculated with the WhatIf? program package (the effective radius of water is 1.4 Å). And on the “aqueous” surface of the membrane a molecule of bound water No. 420 was found. This water molecule is somewhat immersed into a hydrophobic layer and located near the pair Glu194/Glu204. And it is reasonable to consider as the shortest the distance to this water molecule as the distance to the aqueous surface of the membrane. Any projection onto the normal to the membrane of the possible proton pathway on the region under consideration (H2O No. 405 <--> H2O No. 420 is 7.5 Å; H2O No. 403 <--> H2O No. 420 is 6.7 Å; Glu204 <--> H2O No. 420 is 6.2 Å; Glu194 <--> H2O No. 420 is 5.5 Å; H2O No. 404 <--> H2O No. 420 is 4.3 Å, see table) is significantly higher than 2.7 Å1, which is r for SB <--> Asp85, though DeltaV generations on both regions are close to each other. Consequently, the dielectric permeability of the terminal site of the semichannel SB --> external surface of PM is noticeably higher than in the center of the membrane.
1More accurately, this value is slightly higher because in the file 1c3w positions of the atoms are given before the photocycle beginning. However, before the proton transfer from SB onto Asp85 at the K-->L transition the carboxyl group of Asp85 is slightly shifted from the SB [2, 14]. Our evaluations from the ratio of the electric response phases give ~0.4 Å increase in r [2]. By the file 1e0p [15] the increase in r is from 0.5 to 0.8 Å for different atoms of the carboxyl group, and this is in good agreement with our findings and does not change the main conclusion of the present work.We are grateful to Ya. L. Kalaidzidis and S. Spirin for useful discussion and N. V. Khitrin for his help in preparation of the manuscript.
This work was partially supported by the Russian Foundation for Basic Research (the project No. 03-04-49153) and the International Science and Technology Center (No. 2296).
REFERENCES
1.Oesterhelt, D., and Stoeckenius, W. (1971)
Nature New Biol., 233, 149-152.
2.Kalaidzidis, I. V., Kaulen, A. D., Radionov, A. N.,
and Khitrina, L. V. (2001) Biochemistry (Moscow), 66,
1511-1526 (Russ.).
3.Kaulen, A. D. (2000) Biochim. Biophys. Acta,
1460, 204-219.
4.Balashov, S. P. (2000) Biochim. Biophys.
Acta, 1460, 75-94.
5.Lanyi, J. K. (2001) Biochemistry (Moscow),
66, 1477-1482 (Russ.).
6.Oesterhelt, D., and Stoeckenius, W. (1974) Meth.
Enzymol., 31, 667-678.
7.Drachev, L. A., Kaulen, A. D., Khitrina, L. V., and
Skulachev, V. P. (1981) Eur. J. Biochem., 117,
461-470.
8.Lanyi, J. K. (2004) Mol. Membr. Biol.,
21, 143-150.
9.Shishkov, A. V., Ksenofontov, A. L., Bogacheva, E.
N., Kordyukova, L. V., Badun, G. A., Alekseevsky, A. V., Tsetlin, V.
I., and Baratova, L. A. (2002) Bioelectrochemistry, 56,
147-149.
10.Luecke, H., Schobert, B., Richter, H.-T.,
Cartailler, J., and Lanyi, J. K. (1999) J. Mol. Biol.,
291, 899-911.
11.Essen, L.-O., Siegert, R., Lehmann, W. D., and
Oesterhelt, D. (1998) Proc. Natl. Acad. Sci. USA,
95, 11673-11678.
12.Kalaidzidis, I. V., Belevich, I. N., and Kaulen,
A. D. (1998) FEBS Lett., 434, 197-200.
13.Dioumaev, A. K., Richter, H.-T., Brown, L. S.,
Tanio, M., Tuzi, S., Saito, H., Kimura, Y., Needleman, R., and Lanyi,
J. K. (1998) Biochemistry, 37, 2496-2506.
14.Lanyi, J. K. (1993) Biochim. Biophys.
Acta, 1183, 241-261.
15.Royant, A., Edman, K., Ursby, T., Pebay-Peyroula,
E., Landau, E. M., and Neutze, R. (2000) Nature, 406,
645-648.