Inhibition of Naja naja Venom Hyaluronidase by Plant-Derived Bioactive Components and Polysaccharides
K. S. Girish and K. Kemparaju*
Department of Biochemistry, University of Mysore, Mysore 570 006, India; E-mail: kemparaj@rediffmail.com* To whom correspondence should be addressed.
Received February 17, 2005; Revision received March 24, 2005
The inhibitory effect of several bioactive compounds on the activity of hyaluronidase enzyme purified from Naja naja venom was investigated in vitro. Compounds were found to inhibit the hyaluronidase activity dose dependently. Among glycosaminoglycans, heparin, heparan sulfate, and dermatan sulfate showed maximum inhibition compared to chondroitin sulfates. Different molecular forms of chitosan inhibit the enzyme, and inhibition appears to depend on the chain length. In addition, plant-derived bioactive compounds also inhibited the activity of hyaluronidase dose dependently. Among those tested, aristolochic acid, indomethacin, quercetin, curcumin, tannic acid, and flavone exhibited inhibition, with aristolochic acid and quercetin completely inhibiting the enzyme activity. It is concluded that the inhibitors of hyaluronidase could be used as potent first aid agents in snakebite therapy. Furthermore, these inhibitors not only reduce the local tissue damage but also retard the easy diffusion of systemic toxins and hence increase survival time.
KEY WORDS: hyaluronidase, inhibitors, spreading factor, Naja naja venom
Abbreviations: BHT) butylated hydroxytoluene; GAGs) glycosaminoglycans; ECM) extracellular matrix; NDGA) nordihydroguaiaretic acid; NNH1) hyaluronidase from Indian cobra (Naja naja) venom.
Snake envenomation constitutes a medical hazard in most regions of the
world [1]. It is considered as a
subcutaneous/intradermal injection of venom into the prey or human
victims. The pathophysiology of envenomation may include only local
effects (hemorrhage, edema, myonecrosis, and extracellular matrix (ECM)
degradation) or may include systemic effects (neurotoxicity,
myotoxicity, cardiotoxicity, and alterations in hematological systems)
[2, 3]. The latter depends on
the concentration, efficiency, and rate of diffusion of target-specific
toxins from the site of the bite into the general circulation. The
factors involved in the diffusion process are generally referred to as
spreading factors, and they include hemorrhagic matrix
metalloproteases, myotoxins (enzymatic and nonenzymatic), and
hyaluronidases [4-9]. The
hemorrhagic matrix metalloproteases and myotoxins are predominant in
Viperid venoms [10]; in contrast,
hyaluronidase is an invariant factor in all snake venoms and also in
the venoms of bee, spider, scorpion, lizard, stonefish, wasp, and
hornets [8]. The spreading property of this enzyme
is evident from its ability to promote local hemorrhagic effect of a
toxin isolated from Trimeresurus flavoviridis venom [11]. Degradation of hyaluronan in the ECM of local
tissues is presumed to be the key event in the enzyme-mediated
spreading process during snake envenomation. This results in the loss
of integrity of ECM in soft connective tissues surrounding the blood
vessels, which promotes easy diffusion of systemic toxins into their
site(s) of action. Although hyaluronidase potentiates the toxicity of
venom by increasing the influx of systemic toxins, this enzyme has been
largely ignored in snake venoms [5, 8].
Local tissue damage is a continuous process and it continues even after antivenom therapy. Though considered to be the most effective and efficient therapy available, lack of sufficient protection of local tissue is considered to be one of the important limitations associated with antivenom therapy [9, 10]. This limitation might be overcome through characterization of natural and synthetic agents that can neutralize locally acting enzymes/toxins, thereby complementing the action of antivenoms. The inhibition of spreading of toxins is a rate limiting step in the treatment of envenomation, and it is likely that the inhibitors of spreading factors not only minimize local tissue damage but also retard the diffusion of toxins and hence increase the survival time of victims. Inhibitors of hyaluronidase not only prolong survival time, but also prevent the death of experimental animals receiving otherwise lethal doses of Naja kaouthia and Calloselasma rhodostoma venoms [10]. We recently reported the isolation and characterization of isoforms of hyaluronidases from Naja naja venom [8]. Here we have screened different bioactive compounds and polysaccharides for their hyaluronidase inhibitory activity.
MATERIALS AND METHODS
Hyaluronidase (NNH1) enzyme is purified from Indian cobra (Naja naja) venom according to the method of Girish et al. [8]. Hyaluronan, aristolochic acid, ajmaline, reserpine, tannic acid, flavone, curcumin, dexamethasone, indomethacin, ascorbic acid, nordihydroguaiaretic acid (NDGA), n-propyl gallate, butylated hydroxytoluene (BHT), chlorogenic acid, chondroitin sulfates (A and C), heparin, heparan sulfate, and dermatan sulfate were purchased from Sigma (USA). Different forms of chitosans were from Fluka (USA). Streptavidin HRPO-9 and tetramethyl benzidine were purchased from Bangalore Genei Private Limited (India). Biotinylated hyaluronic acid binding protein was from Calbiochem (USA). All other chemicals used were of analytical grade.
NNH1 has been purified to homogeneity using successive chromatography on a Sephadex G-75 and CM-Sephadex C-25 columns. NNH1 was co-eluted with NNH2 on a Sephadex G-75 column, but was separated on CM-Sephadex C-25 column. NNH1 was purified 33-fold and eluted as a single peak with retention time of 43.1 min in reversed-phase HPLC on a Vydac C8 column. NNH1 moved as a sharp band in SDS-PAGE (12.5%) to the same extent under reduced and non-reduced conditions. It revealed a single symmetrical sharp peak at m/z of 70406 in MALDI-TOF mass spectrometry, while the apparent calculated mass was found to be 69 kD by SDS-PAGE (with or without beta-mercaptoethanol) and 74 kD in gel permeation on a Sephadex G-150 column. The homogeneity of NNH1 was further confirmed by the N-terminus sequence analysis of the enzyme, which gave the sequence NEQSTHGAYV [8].
The quantitative estimation of hyaluronidase activity was by ELISA-like assay using biotinylated hyaluronic acid binding protein. Each well of a microtiter plate was coated with human umbilical cord hyaluronan (300 µg/ml) in 0.1 M sodium bicarbonate buffer, pH 9.2, followed by incubation with blocking reagent (5% skimmed milk powder and 2% BSA in 0.01 M phosphate buffered saline (PBS)) and washed three times in PBS. The wells were incubated with hyaluronidase (NNH1) in 0.1 M sodium formate buffer, pH 5.0, containing 0.2 mg/ml BSA with 0.15 M NaCl at 37°C for 10 h. The degraded hyaluronan was washed off with PBS, and undegraded hyaluronan on the wells was determined using biotinylated hyaluronic acid binding protein. The complex was detected with the streptavidin-biotin-peroxidase complex coupled to tetramethyl benzidine/hydrogen peroxide substrate. The absorbance was read at 450 nm as described by the manufacturer (from Bangalore Genei Private Limited). Maximum absorbance was obtained by incubating the hyaluronan-coated wells in the absence of any hyaluronidase. A standard graph was prepared plotting absorbance versus units/mg of bovine testicular hyaluronidase [12].
Plant alkaloids (aristolochic acid, ajmaline, and reserpine), flavonoids (quercetin, flavone, and tannic acid), and anti-inflammatory drug (dexamethasone) were dissolved in dimethyl sulfoxide. Antioxidants (NDGA, BHT, N-propyl gallate, chlorogenic acid, and curcumin) and anti-inflammatory drug (indomethacin) were dissolved in ethanol. Chitosans (different molecular weight forms), antioxidant (ascorbic acid), and glycosaminoglycans (chondroitin sulfates, dermatan sulfate, heparan sulfate, and heparin) were dissolved in 0.2 M sodium acetate buffer, pH 5.0, containing 0.15 M NaCl.
NNH1 (0.75 µg) separately preincubated with alkaloids, flavonoids, antioxidants, and anti-inflammatory drugs in a concentration range from 50-200 µM for 15 min at 37°C in a final volume of 0.1 ml of 0.2 M sodium acetate buffer, pH 5.0, containing 0.15 M NaCl. NNH1 (0.75 µg) and chitosans (0-5 µg) and glycosaminoglycans (GAGs, 0-1 µg) were also preincubated separately in a final volume of 0.1 ml of 0.2 M sodium acetate buffer, pH 5.0, containing 0.15 M NaCl, for 15 min at 37°C. A set of control experiments with inhibitors and/or solvents (buffer/ethanol/dimethylsulfoxide) were performed simultaneously. Reaction was initiated by the addition of preincubated samples (enzyme plus inhibitor) into the wells containing hyaluronan according to the method of Stern and Stern [12]. The percent of inhibition was calculated as:
I, % = 1 - (Amax - Asam)/(Amax - Amin),
where Amax is the absorbance of wells not exposed to hyaluronidase, Amin is the absorbance of wells exposed to NNH1 plus an equal volume of buffer, and Asam is the absorbance of wells exposed to NNH1 plus an equal volume of sample containing inhibitor. The protein content was determined by the method of Lowry et al. [13] using BSA as the standard.
RESULTS AND DISCUSSION
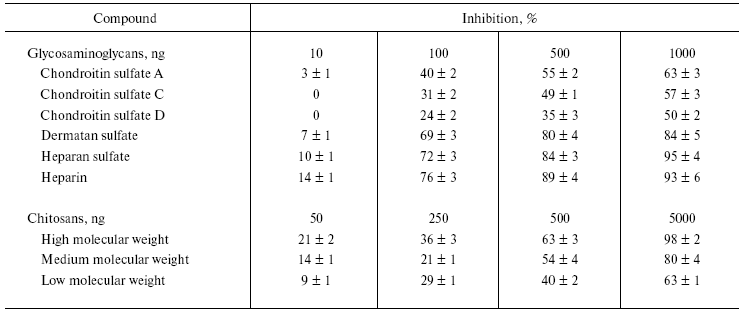
The bioactive compounds such as GAGs, chitosans, alkaloids, flavonoids, antioxidants, and anti-inflammatory drugs inhibited NNH1 activity dose dependently, but to a varied extent (Tables 1 and 2). Among the glycosaminoglycans tested, heparin, heparan sulfate, and dermatan sulfate showed maximum inhibition compared to chondroitin sulfates, which showed moderate inhibition. However, the GAGs, which were resistant to the action of NNH1 [10], were found to inhibit the activity of NNH1. There appears to be electrostatic interaction between the negatively charged GAGs and positively charged NNH1 (basic in property with a pI value of 9.2), and this might prohibit the binding of the substrate hyaluronan resulting in inhibition of the activity. The study of Melo and Ownby [14] demonstrated complex formation between the basic myotoxic PLA2 and the acidic GAG heparin resulted in partial neutralization of myotoxicity of Bothrops jararacussu venom. Diccianni et al. [15] reported the inhibition of a PLA2 enzyme by the negatively charged GAGs and established complex formation with heparin. Further, chitosan, a homopolymer of glucosamine units with beta-1-4 linkages and derived from chitin, also inhibits the enzyme activity and inhibition appears to be directly related to the chain length as inhibition varied as high molecular weight chitosan > medium molecular weight chitosan > low molecular weight chitosan (Table 1). Denuziere et al. [16] reported that chitosan and hyaluronan form a complex due to electrostatic interaction between the NH3+ functions of chitosan and the -COO- groups of hyaluronan. The complex formation makes the substrate not available for the enzyme, and this could be the reason for in vitro inhibition.
Table 1. Effect of various
glycosaminoglycans and different molecular forms of chitosan on the
activity of NNH1
Note: Values are mean ± SEM of five experiments.
Table 2. Effect of various bioactive
compounds (antioxidants, anti-inflammatory substances, flavonoids, and
alkaloids) on the activity of NNH1
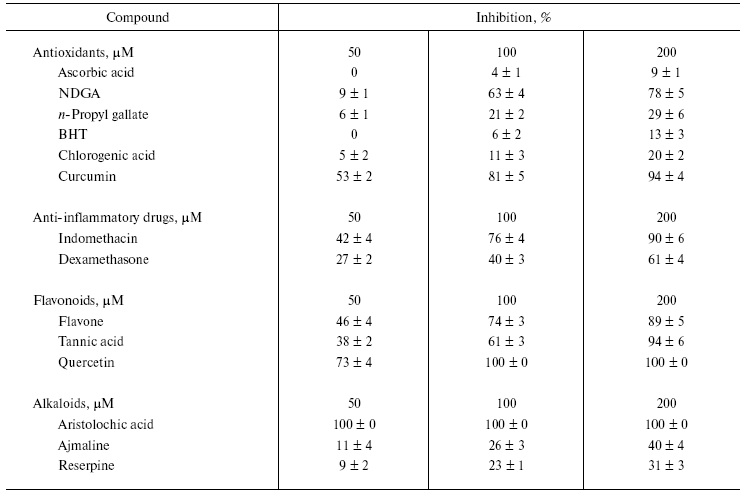
Note: Values are mean ± SEM of five experiments.
Among the antioxidants tested, maximum inhibition was seen with NDGA (78%) and curcumin (91%). In contrast, the other compounds had little or no inhibition. The antioxidant curcumin has been shown to inactivate the neurotoxin isolated from Naja naja siamensis venom [17]. In this study, ascorbic acid showed the least inhibition compared to tested antioxidants (Table 2). In contrast, ascorbic acid and its derivatives inhibit microbial hyaluronan lyase and chondroitinases [18].
Hyaluronan degradation products are found to be potent inducers of inflammatory cytokines [19]. Thus, anti-inflammatory drugs are potential agents for treating inflammatory reactions [20]. NNH1, being associated with the local effects of envenomation leading to the destruction of ECM by degrading hyaluronan and its inhibition, appears to have beneficial effects. Among anti-inflammatory drugs, indomethacin was a more potent inhibitor than dexamethasone. NNH1 is also sensitive to flavonoids, which are biodynamic compounds and are known to posses anti-inflammatory and anti-allergic properties and inhibits PLA2 [20, 21] and hyaluronidase enzymes [22]. Some flavonoids and tannins were reported to prolong the survival time of mice following subcutaneous injection of venom [23]. Among these, quercetin completely inhibits the enzyme activity. In contrast, flavone and tannic acid exhibited similar kind of inhibition pattern with 89 and 94% inhibition, respectively. Some of these compounds (antioxidants, anti-inflammatory substances, and flavonoids) and their synthetic derivatives continue to be used as contraceptives, presumably through their ability to inhibit sperm hyaluronidase activity [24].
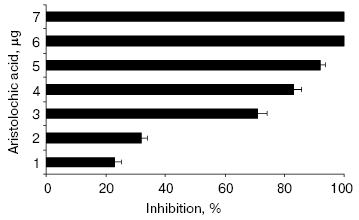
All the alkaloids inhibited NNH1 dose dependently, but to a varied extent. However, among the alkaloids tested, only aristolochic acid, a nitro compound (8-methoxy-6-nitrophenanthro(3,4-D)1,3-dioxole 5-carboxylic acid) was found to abolish the activity of NNH1 (Table 2). The figure shows the dose dependent inhibition of NNH1 by aristolochic acid. The maximum inhibition was observed at a concentration of 58 µM and the molar ratio of enzyme to inhibitor at this point was 1 : 0.0067. Further, the IC50 value determined was found to be 50 µM.
Hyaluronan is a negatively charged, megadalton, multifunctional, non-sulfated glycosaminoglycan with repeating disaccharide units (glucuronic acid-beta-1,3-N-acetyl glucosamine). Hyaluronan holds large amounts of water and metal ions, through its negative charges, conferring viscoelasticity of ECM [25, 26]. In addition, it also maintains the integrity of ECM and gives proper support to connect adjacent cells in tissues. Degradation of hyaluronan by venom hyaluronidase during natural envenomation appears to increase the easy diffusion of venom, which otherwise should have diffused slowly. Thus, hyaluronidase appears to be one of the potential targets and its inhibition not only would reduce the systemic effects but also the local tissue damage.Inhibition of hyaluronidase activity of NNH1 by aristolochic acid. NNH1 (0.75 µg) was preincubated separately with various contents of aristolochic acid (0-7 µg) for 15 min at 37°C and then the mixture was added to hyaluronan coated microtiter plate wells to initiate the reaction. The hyaluronidase activity in the absence of aristolochic acid was taken as 100% activity. Values are mean ± SEM of five experiments
Systemically administered antivenom might be less efficient in reaching the envenomed site resulting in poor or no protection against local tissue damage [9, 10]. Thus, inhibitors of hyaluronidase appear to be likely candidates that not only reduce local tissue damage, but also retard the easy dissemination of systemic toxins and hence increase survival time [6, 9, 10]. In conclusion, the inhibitors of spreading factors may become valuable alternatives to complement the antivenom action and also as first aid agents in snakebite therapy.
We thank Prof. B. S. Vishwanath for his valuable suggestions during this study. K. S. Girish thanks the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for financial assistance.
REFERENCES
1.Chippaux, J. P. (1998) Bull WHO, 76,
515-524.
2.Kini, R. M. (1997) Venom Phospholipase
A2 Enzymes: Structure, Function and Mechanism, J.
Wiley, New York.
3.Aird, S. D. (2002) Toxicon, 40,
335-393.
4.Gopalakrishnakone, P., Ponraj, D., and Thwin, M. M.
(1997) in Venom Phospholipase A2 Enzymes: Structure,
Function, and Mechanism (Kini, R. M., ed.) John Wiley & Sons
Ltd., pp. 287-319.
5.Girish, K. S., Jagadeesha, D. K., Rajeev, K. B.,
and Kemparaju, K. (2002) Mol. Cell. Biochem., 240,
105-110.
6.Anai, K., Sugiki, M., Yoshida, E., and Maruyama, M.
(2002) Toxicon, 40, 63-68.
7.Gutiérrez, J. M., and Ownby, C. L. (2003)
Toxicon, 42, 915-931.
8.Girish, K. S., Shashidhara murthy, R., Nagaraju,
S., Gowda, T. V., and Kemparaju, K. (2004) Biochimie, 86,
193-202.
9.Rucavado, A., Escalante, T., and Gutierrez, J. M.
(2004) Toxicon, 43, 417-424.
10.Yingprasertchai, S., Bunyasrisawt, S., and
Ratanabanangkoon, K. (2003) Toxicon, 42, 635-646.
11.Tu, A. T., and Hendon, R. R. (1983) Comp.
Biochem. Physiol., 76B, 337-383.
12.Stern, M., and Stern, R. (1992) Matrix,
12, 397-403.
13.Lowry, O. H., Rosenbrough, N. J., Farr, A. L.,
and Randall, R. J. (1951) J. Biol. Chem., 193,
265-275.
14.Melo, P. A., and Ownby, C. L. (1999)
Toxicon, 37, 199-215.
15.Diccianni, M. B., Mistry, M. J., Hug, K., and
Harmony, J. A. K. (1990) Biochim. Biophys. Acta, 1046,
242-248.
16.Denuziere, A., Ferrier, D., and Domard, A. (1996)
Carbohydr. Polym., 29, 317-323.
17.Cherdehu, C., and Karlsson, E. (1983) South
Asian J. Trop. Med. Pub. Health, 14, 176-178.
18.Okorukwu, O. N., and Vercruysse, K. P. (2003)
J. Enzyme Inhib. Med. Chem., 18, 377-382.
19.Horton, M. R., Mckee, C. M., Bao, C., Liao, F.,
Farber, J. M., Hodge-DuFour, J., Purae, E., Oliver, B. L., Wright, T.
M., and Noble, P. W. (1998) J. Biol. Chem., 273,
35088-35094.
20.Havsteen, B. (1983) Biochem. Pharmacol.,
32, 1141-1148.
21.Mors, W. B., Nascimento, M. C., Pereira, B. M.
R., and Pereira, N. A. (2000) Phytochemistry, 55,
627-642.
22.Pessini, A. C., Takao, T. T., Cavalheiro, E. C.,
Vichnewski, W., Sampaio, S. V., Giglio, J. R., and Arantes, E. C.
(2001) Toxicon, 39, 1495-1504.
23.Kakegawa, H., Matsumoto, H., and Satoh, T. (1988)
Planta Med., 54, 385-389.
24.Li, M. W., Yudin, A. I., VandeVoort, C. A.,
Sabeur, K., Primakoff, P., and Overstreet, J. W. (1997) Biol.
Reprod., 56, 1383-1389.
25.Matsushita, O., and Okabe, A. (2001)
Toxicon, 39, 1769-1780.
26.Lee, J. Y., and Spicer, A. P. (2000) Curr.
Opin. Cell. Biol., 12, 581-586.