High Molecular Mass Exopolyphosphatase from the Cytosol of the Yeast Saccharomyces cerevisiae Is Encoded by the PPN1 Gene
N. A. Andreeva, T. V. Kulakovskaya*, and I. S. Kulaev
Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia; fax: (495) 923-3602; E-mail: alla@ibpm.pushchino.ru* To whom correspondence should be addressed.
Received April 3, 2006; Revision received May 5, 2006
It has been shown that the high molecular mass exopolyphosphatase localized in cytosol of the yeast Saccharomyces cerevisiae is encoded by the PPN1 gene. This enzyme is expressed under special culture conditions when stationary phase cells are passing on to new budding on glucose addition and phosphate excess. The enzyme under study releases orthophosphate from the very beginning of polyphosphate hydrolysis.
KEY WORDS: polyphosphates, phosphate surplus, exopolyphosphatase, cytosol, yeast, Saccharomyces cerevisiae, PPN1 geneDOI: 10.1134/S0006297906090045
Inorganic polyphosphate is a source of energy, performs regulatory functions, and plays an important role in adaptation of yeast and other microbial cells to changing environment [1, 2]. The cytosol of the yeast Saccharomyces cerevisiae possesses two exopolyphosphatases (polyphosphate-phosphohydrolase, EC 3.6.1.11) splitting Pi from the end of inorganic polyphosphate chain. One of them is encoded by the PPX1 gene [3] and has a molecular mass of ~40 kD, while the other is not encoded by this gene and demonstrates a molecular mass of ~125 to 500 kD during gel-filtration [4, 5]. The above enzymes differ in their activities to polyphosphates of different chain length and expression conditions [4, 5]. The exopolyphosphatase not encoded by PPX1 is expressed in special culture conditions when the stationary phase cells are passing on to new budding on glucose addition and Pi excess [4, 6]. This suggests its specific role in phosphorous metabolism in the yeast.
The aim of this study was to identify the gene encoding the high molecular mass exopolyphosphatase of the cytosol of S. cerevisiae.
MATERIALS AND METHODS
Purification of high molecular mass exopolyphosphatase. Cells of S. cerevisiae VKM Y-1173 were grown in phosphate surplus conditions as described earlier [4, 6]. The purified enzyme was obtained from cytosol fraction according to [5] with the following modification: after adsorption of the protein on heparin-agarose (ICN, USA) and washing with 0.5 M KCl, the resin was again washed with 0.7 M KCl, and only then the exopolyphosphatase was desorbed by 1 M KCl in 25 mM Tris-acetate buffer, pH 7.2, with 0.1% Triton X-100.
Hydrolysis of polyphosphate by purified exopolyphosphatase. The exopolyphosphatase activity was assayed at 30°C by measuring the rate of Pi accumulation [5]. The unit of activity (U) was defined as the amount of the enzyme producing 1 µmol Pi per min. The reaction medium containing 50 mM Tris-HCl buffer, pH 7.2, 200 mM NH4Cl, 0.1 mM CoSO4, 5 mM polyphosphate (chain length of 10-200 phosphate residues; Reakhim, Russia), and purified exopolyphosphatase (34 mU/ml) was used to study the dynamics of polyphosphate hydrolysis. The concentration of polyphosphate is indicated accordingly to the amount of Pi released after hydrolysis of polymer in 1 N HCl for 10 min at 100°C. In different periods of incubation, samples were taken and supplemented with HClO4 to 0.5 N to stop the reaction. Samples were subjected to electrophoresis in 20% polyacrylamide gel in the presence of 7 M urea and the gel was stained with toluidine blue [7]. The Pi released was assayed in the samples as well.
Mass spectrometry. The purified exopolyphosphatase was subjected to electrophoresis in 12.5% polyacrylamide gel in the presence of SDS and the gel was stained with Coomassie blue R-250 [5]. The protein band was cut out, treated with trypsin, and the peptides were mass analyzed by MALDI TOF using an LCO DECAXP mass spectrometer (Thermo Finnigan, USA). The Mascot software (http://www/matrixscience.com/) was used for identification of peptides.
RESULTS
Exopolyphosphatase was purified from cells of S. cerevisiae grown under phosphate surplus, when the enzyme not encoded by the PPX1 gene was predominant in the cytosol [4, 5]. Using a modified purification procedure, an enzyme preparation was obtained demonstrating one protein band of ~33 kD in SDS electrophoresis. The second protein band observed earlier [5] is probably a contamination or degradation product. The specific activity was ~250 U/mg of protein and was higher than as indicated in [5].
The mass spectrometry showed that the amino acid sequence of the protein under study contained several sites corresponding to those of the protein encoded by the PPN1 gene (Swiss Prot: http://kr.expasy.org/enzyme/) [7-9]. The peptides identified in the sequence of exopolyphosphatase under study are indicated in Fig. 1. So, the cytosol exopolyphosphatase not encoded by the PPX1 gene is a product of the PPN1 gene. This is in agreement with the absence of high molecular mass exopolyphosphatase in the cytosol of mutants with inactivated PPN1 gene [10]. Gene PPN1 codes a polypeptide containing 674 amino acid residues with the nominal molecular mass of 78 kD (Swiss Prot: http://kr.expasy.org/enzyme/). The enzyme under study with the subunit molecular mass of ~33 kD is probably a product of mRNA splicing or/and protein processing.
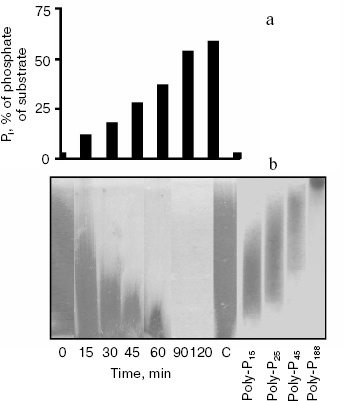
According to literature data, the PPN1 gene codes an endopolyphosphatase (polyphosphate depolymerase EC 3.6.1.10), an enzyme hydrolyzing polyphosphate chains of many hundreds of phosphate residues into shorter chains without Pi release ([7], Swiss Prot: http://kr.expasy.org/enzyme/). Recently it was shown that the product of the PPN1 gene released Pi [9]. Under hydrolysis of polyphosphate by the enzyme under study, the release of Pi appears in the beginning of the reaction, which is characteristic of exopolyphosphatase action (Fig. 2a). Polyphosphate chains are shortening in parallel. By 90 min of the reaction, polyphosphate stained by toluidine blue disappear (Fig. 2b). This indicates that the chain length of remaining polyphosphate becomes less than 10 phosphate residues, and these short-chain polyphosphates are not stained with toluidine blue [2]. During the reaction, the rate of Pi formation decreases due to lower affinity of the enzyme to short-chain polyphosphates. The Km values are 3.5, 75, and 1100 µM for polyphosphates of ~208, 15, and 3 phosphate residues, respectively [5]. However, the enzyme is able to hydrolyze the substrate completely. In 25 h, the reaction mixture contained the same amount of Pi as on the hydrolysis of the used amount of polyphosphate in 1 N HCl at 100°C for 10 min.Fig. 1. Amino acid sequence of the PPN1 gene of Saccharomyces cerevisiae (Swiss Prot: http://kr.expasy.org/enzyme/). The peptides identified by mass spectrometry of trypsin hydrolyzate of high molecular exopolyphosphatase are underlined.
Fig. 2. Hydrolysis of polyphosphate by purified exopolyphosphatase encoded by the PPN1 gene. a) Pi formation; b) electrophoresis of polyphosphates in 20% polyacrylamide gel, staining with toluidine blue. C, control, polyphosphate was incubated in reaction mixture without the enzyme for 120 min. PolyP15, polyP25, polyP45, and polyP188 are polyphosphate markers (Sigma and Monsanto, USA), the numbers show average chain lengths.
DISCUSSION
The present work demonstrates for the first time that high molecular mass exopolyphosphatase of S. cerevisiae cytosol is encoded by the PPN1(PHM5) gene. The enzyme appears in the cytosol during the transition of cells from stationary growth phase to new budding on glucose addition and Pi excess [4, 6]. Previously it was believed that the enzyme encoded by this gene is localized in vacuoles, though it was purified from the whole cell homogenate [7, 9]. Indeed, the exopolyphosphatase activity in the vacuoles decreases in the mutant with inactivated PPN1 gene [11]. The similarity of high molecular mass cytosol exopolyphosphatase and vacuolar exopolyphosphatase in many properties should be noted [5, 12]. At the same time, exopolyphosphatase activity is absent in mitochondrial membranes and nuclei of the above mutant [11]. The high molecular mass exopolyphosphatase in the cytosol of the PPN1 mutant disappears as well [10]. These data point to multiple localization of PPN1 gene products. The PPN1 gene sequence possesses domains characteristic of potential cytoplasmic and vacuolar proteins and also a signal anchor for membrane proteins (Swiss Prot: http://kr.expasy.org/enzyme/). Different yeast cell compartments probably possess the PPN1 gene products as a result of alternative splicing or protein processing. It should be noted that the PPN1 gene product purified in our study contains a peptide (580 to 605 amino acid residues) that has not been observed in the amino acid sequence of endopolyphosphatase [9]. The formation of different proteins as products of the same gene by alternative mRNA splicing, or protein processing, is widespread in eukaryotes [13]. In some cases, these proteins differ in their localization in the cell [14].
Multiple localizations of PPN1 gene products may result in their pleiotropic action. Upon its inactivation, the viability of yeast cells in the stationary growth stage decreases [8], the formation of functionally active mitochondria is disturbed [15], and chain lengths of cellular polyphosphates increase [8, 11]. This suggests an important role of the PPN1 gene in phosphorous metabolism in the yeast.
We thank E. Kulakovskaya for her help with polyphosphate electrophoresis.
This work was supported by the Russian Foundation for Basic Research (grant 05-04-48175) and by the grant of support of leading scientific schools of Russia (NSh-4318.2006.4).
REFERENCES
1.Kulaev, I. S. (1979) The Biochemistry of
Inorganic Polyphosphates, Wiley, New York.
2.Kulaev, I. S., Vagabov, V. M., and Kulakovskaya, T.
V. (2004) The Biochemistry of Inorganic Polyphosphates, Wiley,
New York.
3.Wurst, H., Shiba, T., and Kornberg, A. (1995) J.
Bacteriol., 177, 898-906.
4.Andreeva, N. A., Kulakovskaya, T. V., and Kulaev,
I. S. (2001) Biochemistry (Moscow), 66, 147-153.
5.Andreeva, N. A., Kulakovskaya, T. V., and Kulaev,
I. S. (2004) Biochemistry (Moscow), 69, 387-393.
6.Kulakovskaya, T. V., Andreeva, N. A., Trilisenko,
L. V., Suetin, S. V., and Kulaev, I. S. (2005) Biochemistry
(Moscow), 70, 980-985.
7.Kumble, K. D., and Kornberg, A. (1996) J. Biol.
Chem., 271, 27146-27151.
8.Sethuraman, A., Rao, N. N., and Kornberg, A. (2001)
Proc. Natl. Acad. Sci. USA, 98, 8542-8547.
9.Shi, X., and Kornberg, A. (2005) FEBS Lett.,
579, 2014-2018.
10.Lichko, L., Kulakovskaya, T., and Kulaev, I.
(2004) Biochim. Biophys. Acta, 1674, 98-102.
11.Lichko, L. P., Kulakovskaya, T. V., Pestov, N.
A., and Kulaev, I. S. (2006) Biosci. Rep., DOI
10.1007/s10540-006-9003-2.
12.Andreeva, N. A., Kulakovskaya, T. V., and Kulaev,
I. S. (1998) FEBS Lett., 429, 194-196.
13.Stetelfeld, J., and Ruegg, M. A. (2005)
Trends. Biochem. Sci., 30, 515-521.
14.Mueller, J. C., Andreoli, C., Prokisch, H., and
Meitinger, T. (2004) Mitochondrion, 3, 315-325.
15.Pestov, N. A., Kulakovskaya, T. V., and Kulaev,
I. S. (2005) FEMS Yeast Res., 5, 823-828.