hhLIM Is Involved in Cardiomyogenesis of Embryonic Stem Cells
Bin Zheng1, Jin-Kun Wen1,2*, and Mei Han1
1Hebei Laboratory of Medical Biotechnology, Institute of Basic Medicine, Hebei Medical University, Shijiazhuang 050017, China2Department of Biochemistry and Molecular Biology, Institute of Basic Medicine, Hebei Medical University, No. 361, Zhongshan East Road, Shijiazhuang 050017, China; fax: 86 (0) 311-6696826; E-mail: wjk@hebmu.edu.cn
* To whom correspondence should be addressed.
Received October 25, 2004; Revision received July 9, 2005
Proteins of the LIM family play important roles in a variety of fundamental biological processes including cell lineage specification and organ development. Here we examined the function in cardiogenesis of a new member of the LIM family, hhLIM, by molecular analysis of early stages of cardiomyocyte differentiation in hhLIM-deficient P19 cell line and P19 cells stably overexpressing hhLIM. The results indicate that hhLIM is a potent transcriptional activator of several cardiac muscle-specific genes. Inhibition of hhLIM expression by antisense transcripts can interfere with expression of cardiac muscle genes and block development of beating cardiomyocytes in P19 embryonic stem cells. Overexpression of hhLIM in P19 cells can enhance expression of cardiac marker genes Nkx2.5 and GATA-4 and potentiate development of cardiomyocyte-like morphology. These findings suggest that, in addition to its role in activation of the cardiac genetic program, hhLIM may be the nuclear target of inductive factor for precardiac cells.
KEY WORDS: hhLIM protein, heart embryonic stem cells, cardiomyocyte differentiationDOI: 10.1134/S0006297906130128
Abbreviations: DMSO) dimethylsulfoxide; EC) embryonal carcinoma; RA) retinoic acid.
In higher vertebrates, heart formation is a complex process that starts
at early stages of embryogenesis, prior to the end of gastrulation,
with commitment of anterior lateral plate mesoderm cells to the
cardiogenic lineage. These committed precursors subsequently
differentiate into cardiomyocytes, and the heart develops principally
through proliferation of the differentiated myocytes. A delicate
balance between cell differentiation, proliferation, and apoptosis is
crucial for proper organogenesis, and cell-specific transcription
factors, which play pivotal roles in cell fate determination, are
critical for understanding the molecular pathways underlying cell
behavior during organ formation [1]. Unfortunately,
little is known about the molecular events, including expression of
candidate genes, which are required for either cardiac muscle cell
differentiation or heart morphogenesis, although gene inactivation
studies have been started to identify genes that may be involved in the
latter process.
Homeodomain-containing protein Nkx2.5 is a factor required for heart development and the earliest known marker of vertebrate heart development [2]. Zinc-finger-containing protein GATA-4 also plays an important role in early cardiogenesis. GATA-4 null mice display a severe defect in formation of the cardiac tube, which is required for migration and folding morphogenesis of precardiogenic splanchnic mesodermal cells [3].
The LIM protein contains a zinc finger structure that is present in several protein types consisting of several LIM domains [4]. Proteins containing LIM domains have been discovered to play important roles in a variety of fundamental biological events including cytoskeleton organization, cell lineage specification, and organ development. Recently, a new gene named hhLIM (human heart LIM protein, also known as myogenic factor LIM3) (GenBank AF121260) was cloned by three element PCR-select cDNA subtraction [5]. It belongs to the LIM family since it has the typical LIM domain. We have reported that hhLIM protein plays a role as an effector of the hypertrophic response [6]. For example, the recent study showed an increase in hhLIM RNA level or transcriptional activity in pressure-induced cardiac hypertrophy. In addition, transfection assay in cardiac myocytes showed that hhLIM could enhance the cell growth [7, 8]. During embryogenesis, hhLIM transcripts are detected in cardiac cell progenitors, suggesting that expression of the hhLIM gene is tightly linked to cardiomyocyte specification.
To determine the role of hhLIM in cardiac muscle differentiation, we utilized the pluripotent embryonal carcinoma (EC) P19 cell line as an in vitro model system. In this system, we found that hhLIM expression was restricted to cells destined to cardiac muscle differentiation. Moreover, inhibition of hhLIM expression with hhLIM antisense constructs specifically blocked cardiac muscle gene expression and inhibited the appearance of beating cardiac cells, indicating that hhLIM is involved in differentiation of cardiac muscle cells.
MATERIALS AND METHODS
Cell culture and in vitro differentiation. P19 cells were obtained from Peking Union Medical College. Cells were grown in a minimum essential medium (Gibco, USA) supplemented with 10% fetal bovine serum. 105 cells per ml were grown in suspension in bacteriological Petri dishes in medium containing 1 mM retinoic acid (RA; Sigma, USA) and 0.8% dimethylsulfoxide (DMSO; Sigma) for neuronal and cardiac muscle differentiation, respectively. After 4 days, the cells adhered to each other to form small aggregates, which were then plated into tissue culture-grade plastic dishes and maintained without any drug. Days of differentiation were numbered consecutively after the first day of aggregation.
Transfection. Undifferentiated P19 cells were maintained in a minimum essential medium and transiently transfected using ESCORTTM Transfection Reagent (Sigma). For stable transfections, 1 µg of hhLIM antisense expression vector and 1 µg of hhLIM expression vector previously described were added to P19 cells, respectively. Selections were conducted at 800 µg of G418 (Gibco) per ml.
RNA extraction and analysis. Total cellular RNA was extracted by the thiocyanate-phenol-chloroform method. Transcripts were detected by semi-quantitative reverse transcription-PCR (RT-PCR). The PCR conditions were 94°C for 1 min, 54 to 61°C for 1 min depending on the melting temperature (Tm) of the primers, and 72°C for 1 min, repeated for 30 cycles. The quantity of cDNA used in each PCR reaction was chosen in the linear range of the signal according to a dose-response curve carried out for each set of primers. The following pairs of primers were designed, hhLIM: (5'-GCTGTCTCAGCACAGACA-3', 5'-CACAGATGGCACAGCGGA-3´); GATA-4: (5'-TCATTAAGCCTCAGCGCCGCCT-3', 5'-CTCACCCTCGGCATTACGAC-3'); Nkx2.5: (5'-ACCTGTCTGATCTGTCCTCT-3', 5'-TGCCTTCTGGGTTGTCTGCC- 3').
The level of transcript for the constitutive housekeeping gene product, GAPDH, was quantitatively measured for each sample to control differences in RNA concentrations. The data were analyzed by Bio 1D software (Kodak, USA).
Western blot analysis. Cells were harvested and protein extracts were prepared in RIPA buffer (1% NP40, 1% SDS, 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 10% glycerol, 1 mM NaVO3, 1 mM PMSF) as described previously [8, 9]. Protein concentration was determined by a modified Lowry protein assay. Protein (50 µg per sample) was separated by polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and blocked in 5% milk/TTBS (0.5 M NaCl, 0.05% Tween 20, 50 mM Tris-HCl, pH 7.5). Membranes were incubated overnight at 4°C with hhLIM antibody (a gift from Dr. K. H. Chen) and then incubated with an alkaline phosphatase-conjugated secondary antibody. Bands were detected by chemiluminescence (Visa ECF, Amersham Pharmacia Biotech).
Immunocytochemistry. P19 cell aggregates were dispersed mechanically and plated in 35-mm-diameter culture dishes. After cells attached (2 to 3 h), they were directly fixed with 4% paraformaldehyde for 30 min at room temperature. The cells were blocked with a solution of 1% BSA and then incubated overnight at 4°C with antibody against hhLIM (1 : 100) (a gift from Dr. K. H. Chen). TRITC conjugated secondary antibody was used at 1 : 100 for 2 h at room temperature. Stained cells were observed with a Leica DMRE confocal laser-scanning microscope (Leica Microsystems, Germany). All figures were made using Adobe Photoshop 5.5 (Adobe Systems Inc., USA).
Statistics. Results are reported as the mean values ± SEM. Unpaired Student's t-test was performed for statistical analysis.
RESULTS
hhLIM expression is restricted to differentiating cardiac cells. When aggregated and treated with different drugs, P19 cells differentiate into a variety of cell types. RA treatment induces P19 cell differentiation into neurons and other neuroectodermal derivatives, whereas DMSO treatment leads to development of beating cardiac cells by 7 days in culture. hhLIM transcripts were detected only in DMSO-treated P19 cell cultures which contain beating cardiac muscle cells (Fig. 1), but not in undifferentiated cultures or in cultures differentiating into neurons (data not shown).
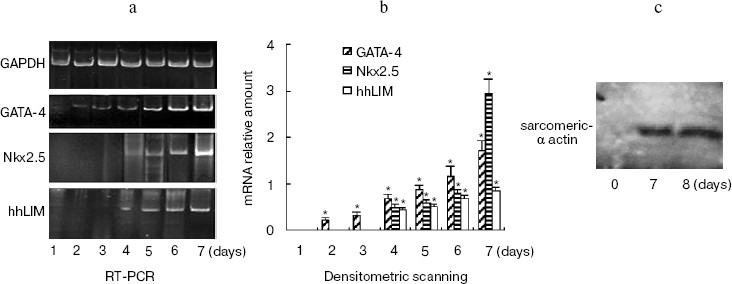
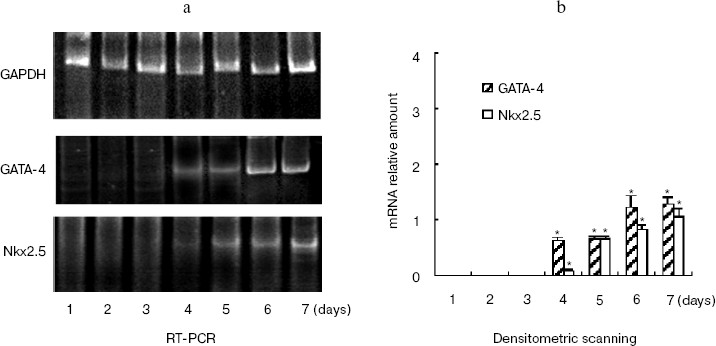
The time course of gene expression during P19 cell differentiation into cardiac cells was established using RT-PCR. Figure 2a shows that GATA-4 appears to be expressed at early stages of P19 cell cardiac differentiation, and there was a steady increase over 7 days of the experiment. The profile of Nkx2.5 and hhLIM expression was similar to that of GATA-4, and the appearance of beating cardiac cells was observed on day 7. Western blot showed that cardiomyocyte marker protein sarcomeric alpha actin expression was detected on day 7 and day 8 after DMSO treatment (Fig. 2c).Fig. 1. Expression of hhLIM during P19 cell differentiation into cardiomyocytes. P19 cells were grown in a minimum essential medium supplemented with 10% fetal bovine serum. Cells were grown in suspension in bacteriological Petri dishes in medium containing 0.8% DMSO for cardiac muscle differentiation. hhLIM expression was analyzed by Western blot on different days of P19 cell differentiation induced by DMSO.
Ectopic expression of hhLIM potentiates cardiomyocyte formation.Fig. 2. Expression of cardiac marker genes during P19 cell differentiation into cardiomyocytes. Differentiating P19 cells were harvested at indicated times (1 to 7 days) after DMSO treatment. hhLIM, GATA-4, Nkx2.5, and GAPDH expressions were analyzed by RT-PCR (a). Similar results were obtained in two independent differentiation experiments and standardized using GAPDH, and densitometric scanning data are shown (b). The sarcomeric alpha actin was detected by Western blot (c) on day 7 and day 8 after DMSO treatment. * p < 0.05 compared with the expression of day 1.
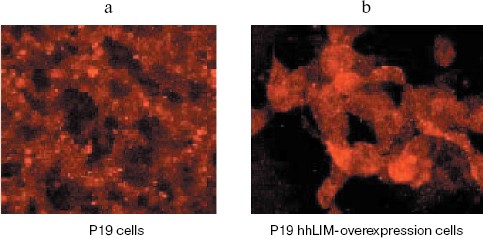
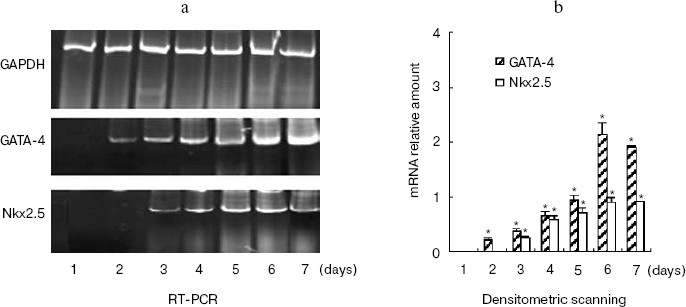
To confirm the role of hhLIM in cardiomyocyte differentiation, we performed gain-of-function experiments by overexpressing hhLIM in P19 cells. P19-hhLIM-expressing cells did not show morphological or biochemical evidence of cardiomyogenesis (data not shown). Beating cardiomyocytes were evident in P19 cells upon aggregation induced by DMSO (Fig. 3a, see color insert, p. 6). However, when hhLIM-expressing clones were aggregated and treated with DMSO, beating foci appeared earlier, and their number was markedly increased (Fig. 3b, see color insert, p. 6). Ectopic expression of hhLIM did not markedly alter cell number in these experiments but had a beneficial effect on cell aggregates that were especially obvious in the absence of DMSO. Indeed, when P19 cells were aggregated without any inducer, they showed poor survival and, by day 5-6, most cells were detached in the media. Cell viability in the absence of inducer was rescued in P19 clones ectopically expressing hhLIM, which showed that overexpression of hhLIM protein promoted P19 cell survival (data not shown). Consistent with the earlier appearance of beating cells, accelerated cardiogenesis was evidenced at the mRNA level by the earlier expression of cardiac marker gene Nkx2.5. Additionally, increased amount of GATA-4 mRNA was obvious by day 6-7 in hhLIM-expressing cells compared to normal P19 cells treated with DMSO (Fig. 4). Together, the data are consistent with an important role of hhLIM in differentiation of mesodermal cells along the cardiogenic pathway.
Fig. 3. Immunohistochemistry for detecting hhLIM protein. P19 cells were grown in a minimum essential medium and transfected with hhLIM expression plasmid. Then the cells were induced by DMSO. At 6 days after DMSO treatment, hhLIM protein was analyzed by immunohistochemistry.
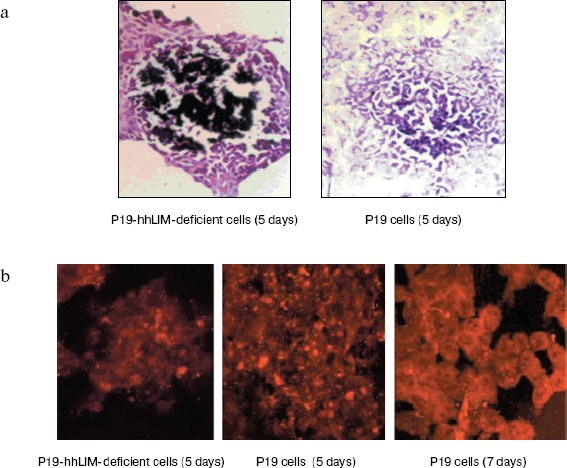
hhLIM expression is required for development of cardiac muscle cells. The role of hhLIM in establishment of the cardiac phenotype was directly tested in stable P19-derived cell lines, which express the antisense hhLIM sequence. These functional hhLIM null cell lines (P19-hhLIM-deficient cells) were analyzed for the ability to be differentiated into cardiac myocytes or neuronal cells. The presence of the hhLIM antisense sequence had no effect on ability of P19 cells to undergo RA-induced differentiation into neuroectoderm derivatives (data not shown). However, a pronounced phenotypic effect was observed when transfectants were treated with DMSO for cardiac muscle differentiation. Indeed, the hhLIM antisense clones differentiated very poorly when being induced with DMSO; this was evident by 5 to 6 days following DMSO treatment, whereas aggregates of control P19 cell clones were well spread and cells looked healthy, with very few undifferentiated-like cells remaining. In contrast, aggregates of hhLIM antisense clones treated with DMSO were compact and poorly spread and contained a large proportion of undifferentiated EC-like cells; on day 7, these aggregates appeared to disintegrate, and many dead cells were floating; there was never evidence of cardiomyocyte-like morphology, and no beating cells were ever detected (Fig. 5, see color insert, p. 6). These observations suggest that P19 cells deficient in hhLIM cannot differentiate into cardiomyocytes and raise the possibility that hhLIM is required for development of cardiac cells. In contrast, overexpression of hhLIM in P19 cells appeared to enhance cardiac differentiation, as determined on the basis of both morphology and expression of cardiac marker genes. However, aggregation was still required for development of beating cardiomyocytes, suggesting that hhLIM alone may not be sufficient to commit undifferentiated cells to a cardiac fate. The morphological studies were corroborated by RNA analysis of several cardiac markers. Transcripts for the cardiac muscle-specific Nkx2.5 and GATA-4 gene was later in total RNA prepared from hhLIM antisense clones treated with DMSO (Fig. 6). These data are consistent with the absence of differentiated cardiomyocytes in hhLIM null P19 clones and suggest that hhLIM is a key activator of the cardiac genetic program and a necessary factor for cardiac cell differentiation.Fig. 4. P19 cell differentiation induced by DMSO was stimulated by hhLIM overexpression. P19-hhLIM-overexpression cells were induced by DMSO. GATA-4, Nkx2.5, and GAPDH expressions were analyzed by RT-PCR on different days of P19 cell differentiation induced by DMSO (a). Similar results were obtained in two independent differentiation experiments and standardized using GAPDH, and densitometric scanning data are shown (b). * p < 0.05 compared with the expression of day 1.
Fig. 5. Phase-contrast photomicrograph of cultures showing morphologies of P19 cells and P19-hhLIM-deficient cells. P19-hhLIM-deficient cells were induced for cardiac differentiation by DMSO treatment. Morphologies of P19 cells and P19-hhLIM-deficient cells were detected by hematoxylin-eosin staining (a) and immunohistochemistry (b), respectively.
Fig. 6. P19 cell differentiation induced by DMSO was inhibited by hhLIM antisense RNA. P19 cells were transfected with hhLIM antisense plasmids. Then the cells were induced by DMSO for cardiac muscle differentiation. GATA-4, Nkx2.5, and GAPDH expressions were analyzed by RT-PCR on different days of P19 cell differentiation induced by DMSO (a). Similar results were obtained in two independent differentiation experiments and standardized using GAPDH, and densitometric scanning data are shown (b). * p < 0.05 compared with the expression of day 1.
DISCUSSION
The heart is the first organ to form in mammals. Cardiogenic lineages originate from paired regions of anterior lateral mesoderm, the cardiac crescent, soon after gastrulation and develop into parallel cardiac primodia that fuse to form the primitive heart tube along the ventral midline of the embryo [10]. Several recent studies have revealed cis-regulatory elements that direct cardiac development events. The homeodomain-containing protein Nkx2.5 is the earliest known marker of vertebrate heart development. Nkx2.5 is expressed concomitant with cardiac specification and is a known marker of the cardiac lineage [11]. Zinc-finger-containing protein GATA-4 is a cardiac muscle-specific member of the GATA family of transcription factors, which plays a critical role in cell differentiation. GATA-4 is expressed with Nkx2.5 in developing heart. In fact, GATA-4 and Nkx2.5, two of the earliest markers of precardiac cells, are essential for heart development [10]. Recently, a new gene named hhLIM was cloned. It belongs to the LIM family since it has the typical LIM domain. In recent years, accumulating results have continued to attribute essential functions to LIM proteins in a variety of different biological processes. But little is known about the role of the new gene hhLIM. We have found that nuclear accumulation of hhLIM is associated with induction of cell hypertrophy. The interaction of hhLIM with GATA-4 and Nkx2.5 could regulate the cardiac hypertrophy responses. We also found that the pattern of hhLIM expression overlaps with that of GATA-4 and Nkx2.5 in the heart-forming region in various species [12].
The analysis of early cardiac markers has allowed the establishment of a cascade of transcription factor expression, which will undoubtedly prove useful in analyzing molecular events underlying cardiac phenotypes. In this respect, it is worth mentioning that the P19 system is especially amenable to establishing the time course of appearance of cardiac transcription factors because it is more homogenous than the other available in vitro model of cardiogenesis consisting of EC-derived embryoid bodies. The data obtained in this system using both gain and loss of hhLIM function show that hhLIM is an inducible cardiac transcription factor that plays a key role in cardiomyocyte differentiation. The similarity between hhLIM function in cardiac cell differentiation and the crucial role of MLP (muscle LIM protein) in development and maturation of erythroid cells is especially interesting given that in both cases tissue-restricted LIM factors are involved in early differentiation events that are necessary for development of the primitive cardiovascular system of the embryo [13]. In addition, molecular analysis of cardiac muscle differentiation has been hampered by the lack of an in vitro culture system. The in vitro approach used here demonstrates that P19 cells provide a useful system for further dissecting molecular networks involved in the early developmental decisions underlying cardiogenesis. Our in vitro study suggests that hhLIM is crucial for cardiogenesis and is therefore likely to be essential for normal embryonic development. Thus, hhLIM may share conserved roles in lineage development and terminal cell differentiation. At this stage, it is noteworthy that studies so far show that hhLIM plays important roles in transcription and differentiation of cells derived from the lateral plate mesoderm, particularly cells of the cardiac lineages.
Although hhLIM overexpression enhanced cardiogenesis, cell aggregation was required for development of terminally differentiated cardiomyocytes. In fact, ectopic expression of hhLIM led to only subtle changes in untreated monolayer P19 cells; these included regulation of cell markers such as transcription factors GATA-4 and Nkx2.5. However, in hhLIM-overexpressing cells, cardiac differentiation could be achieved in the presence or absence of DMSO, suggesting that hhLIM may relay diffusible and/or cell contact-generated signals required for cardiomyocyte differentiation.
This work was supported by the Major State Basic Research Development Program of the People's Republic of China (G2000056905), National Natural Science Foundation of the People's Republic of China (No. 30300132), and Hebei Natural Science Foundation of the People's Republic of China (No. 2004000644).
REFERENCES
1.Grepin, C., Nemer, G., and Nemer, M. (1997)
Development, 124, 2387-2395.
2.Schwartz, R. J., and Olson, E. N. (1999)
Development, 126, 4187-4192.
3.Charron, F., and Nemer, M. (1999) Semin. Cell
Dev. Biol., 10, 85-91.
4.Bach, I. (2000) Mech. Dev., 91,
5-17.
5.Chen, K. H., Zhou, Z. Z., Zhang, J. F., and Zhou,
A. R. (2000) Chin. J. Biochem. Mol. Biol. [in Chinese],
16, 295-300.
6.Zheng, B., Wen, J. K., Han, M., and Zhou, A. R.
(2002) Progr. Biochem. Biophys. [in Chinese], 29,
911-914.
7.Zheng, B., Wen, J. K., and Han, M. (2003)
Biochemistry (Moscow), 68, 650-657.
8.Zheng, B., Wen, J. K., Han, M., and Zhou, A. R.
(2004) Biochim. Biophys. Acta, 1690, 1-10.
9.Wen, J. K., and Han, M. (2000) Biochemistry
(Moscow), 65, 1376-1379.
10.Grepin, C., Robitaille, L., Antakly, T., and
Nemer, M. (1995) Mol. Cell Biol., 15, 4095-4102.
11.Zhu, W., Shiojima, I., Hiroi, Y., Zou, Y.,
Akazawa, H., Mizukami, M., Toko, H., Yazaki, Y., Nagai, R., and Komuro,
I. (2000) J. Biol. Chem., 275, 35291-35296.
12.Zheng, B., Wen, J. K., Han, M., and Zhou, A. R.
(2003) Progr. Biochem. Biophys. [in Chinese], 30,
67-71.
13.Jiang, Y., Drysdale, T. A., and Evans, T. (1999)
Dev. Biol., 216, 57-71.