|
REVIEW: Reorganization of Molecular Morphology of Epitheliocytes and Connective-Tissue Cells in Morphogenesis and CarcinogenesisJ. M. VasilievBlokhin Cancer Research Center, Russian Academy of Medical Sciences, Kashirskoe Shosse 24, 115478 Moscow, Russia; E-mail: yuvasiliev@yahoo.com, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia |
Received December 21, 2007
Complete and incomplete transitions of epitheliocytes into cells of mesenchymal type, so-called epithelial-mesenchymal transitions (EMT), take place in many types of normal morphogenesis and in epithelial carcinogenesis. Connective tissue cells (fibroblasts) also undergo considerable morphological changes during normal morphogenesis and carcinogenesis, but their dynamics are less known. It is suggested that EMT and fibroblast dynamics may have some common step that is some united precursor cell type. The program for normal EMT can be activated in the course of multistep progression of epithelial carcinogenesis; this activation can be supported by cell selection as it provides a basis for dissemination of neoplastic cells from original tumor.
KEY WORDS: microtubule, microfilament, juvenile fibroblast, mature fibroblast, malignant transformationDOI: 10.1134/S0006297908050052
It has been known for more than a century that morphological transition is a characteristic feature of carcinogenesis. All pathohistological diagnosis of tumors in clinics is based on ascertainment of these changes. A spectrum of morphological methods applied towards analysis of cells has therewith experienced a fundamental transformation in recent decades. We now have the ability to detect a multiplicity of biological molecules, to localize them in both fixed and living cells, and analyze their dynamics in the living cell, culture, and organism. So, it is quite in order to speak about a dynamic molecular morphology of cells.
We will attempt to consider the most important problem in cell biology and oncology--the problem of interrelationship between two main Metazoan tissue types: epithelium and internal tissues (mesenchyme). Transitions between these tissue types are found to occur both in ontogenesis and carcinogenesis.
EPITHELIAL-MESENCHYMAL TRANSITION (EMT) IN NORMAL
MORPHOGENESIS
The epithelial-mesenchymal transition (EMT) is one of the main morphogenetic processes involved, as a fundamental part, in virtually all Metazoan embryogeneses. The genesis of Metazoa with several germ layers from a single-layer colonial Protozoa is also a product of complete EMT, according to Mechnikov's theory, or incomplete EMT, according to Haeckel's theory [1]. Either complete or incomplete EMT underlies gastrulation in various phyla of animals. In vertebrates, the complete EMT underlies the first stage of neural crest evolution [2], whereas incomplete EMT underlies angiogenesis and many variants of organogenesis. A development of an experimental model for EMT in cell culture has brought a great advance in EMT study. The protein called Hepatocyte Growth Factor/Scatter Factor (HGF/SF) added to the epithelium culture induces EMT: islets of tight-knit discoid epitheliocytes disjoin to many elongated motile cells of mesenchymal type, which actively move individually [3]. The protein HGF/SF is produced in vivo by many cells of mesenchymal origin and possibly plays a role in morphogenetic reorganizations of adjacent epithelial cells. Complete EMT is observed upon the influence of HGF/SF on epithelial islets growing in ordinary flat-bottomed cell culture flasks. The same protein, when influencing the culture of the same cells growing in flasks with collagen gel (a net of matted collagen fibers), causes the growth of epithelium to form bands and tubes [3]. In this case, cells do not detach completely, but contacts between them diminish, the cells stretch, and the marginal cells move ahead, thus elongating the entire cell band. It remains unclear what factor determines the type, complete or incomplete, of EMT. Here we are apparently dealing with a type of interaction of cells with an underlying matrix, for example, with the number of focal integrin contacts.
To date, many steps of the HGF/SF-activated signaling pathway cascade leading to complete EMT are studied in detail [4]. A specific HGF/SF receptor, the protein Met, is known. Activation of specific Snail proteins, as well as other proteins, has been demonstrated at intermediate EMT steps. When EMT occurs, many proteins specific for epithelia disappear, such as keratin group proteins, from which intermediate filaments typical of all epithelia are built. These keratins are substituted by vimentin, the intermediate filament protein typical of all cells of mesenchymal origin. By analogy, E-cadherin (a protein of epithelial cell-cell adherens junctions) is substituted by N-cadherin (a protein of connective-tissue adherens junctions) [4]. Morphology of these contacts also changes. Tight junctions and desmosomes completely disappear together with their characteristic proteins, such as ZO-1, desmoplakins, and many others.
So far little is known about rearrangements of actin cytoskeleton in EMT. Circular marginal actin-myosin bundles disappear at early stages of EMT. Nevertheless, a small amount of thin direct actin-myosin bundles persists in EMT. Mature cell-extracellular matrix contacts mediated by integrins also disappear. Very little is known about mechanisms of these cytoskeleton and adhesion structure rearrangements. It would appear reasonable that cell-cell contacts are early targets of a cascade initiated by activation of Met. However, this hypothesis lacks support, because E-cadherin-based adherens junctions are maintained through late EMT stages [5]. One of the early manifestations of the effect of HGF/SF on sensitive epithelium is induction of actin filament polymerization sites on the edge of the cell. This may be somehow associated with dissociation of cell-cell contacts, but it is not studied in detail. There is scarcely any data on alterations in the microtubule system in EMT. No apparent changes of microtubules are observed in EMT.
EMT IN EPITHELIAL CARCINOGENESIS
In the beginning of the last century, researchers from the London Institute of Cancer found that epithelial tumors of murine mammary glands (adenocarcinomas), when passed several times from one mouse to another, often change their morphology and turn into sarcomatous tissue composed of connective tissue-type cells [6]. This phenomenon was commonly assigned to malignization of tumor stroma, but now it is arguable that the EMT of tumor epithelium cells is concerned, because such “sarcomatous” transformation repeatedly occurs in cell culture lacking stroma cells. EMT is therewith induced by various agents, including tumorigenic viruses and chemical carcinogens [7]. A very convenient model for such EMT is one-stage transformation caused by a transfection of the N-ras oncogene into the cells of IAR epithelial strain [8]. The strains obtained from these cells strictly correspond in morphology to the cells at middle and late stages of phenotypical EMT caused by HGF/SF (most commonly these are elongated fibroblast-like cells lacking E-cadherin). These cells do not contain large actin-myosin bundles and do not form mature integrin-based focal contacts with matrix, but contain thin actin bundles and small focal integrin complexes. Many of these cell strains cause tumor formation after transplantation [7], but the question remains unclear on association of tumorigenicity with degree of loss of epithelial markers.
Most epithelial malignant tumors (carcinomas) of humans and animals demonstrate epithelial features, thus allowing diagnosis on their tissue origin. A presence of cysts, bands, tubules, and other related structures in these tumors allows their morphological comparison with the structures induced by HGF/SF in epithelial cultures growing in a collagen gel. In other words, there are grounds to believe that the cells of these tumors are in the state of “statuesque” incomplete EMT.
CHANGES IN MOLECULAR MORPHOLOGY OF FIBROBLASTS (MESENCHYMOCYTES)
IN NORMAL MORPHOGENESIS
The cultured fibroblast is a morphological antetype of all variants of mesenchymal-origin cells (osteoblasts, chondroblasts, and many others) and related cells of neuronal origin (gliocytes). Such fibroblasts are planar cells, which are spread on a flat endless substrate [9]. These cells acquire a cylindrical form on bounded substrate area or on non-flat or low-adhesive substrates. A broad lamella at one of the ends of elongated cells has the active edge, on which polymerization of actin microfilaments and formation of flat pseudopodia (lamellipodia) occur. A complex of proteins involved in this polymerization (Arp 2/3, WASP, VASP, etc.) serves as a marker for the active edge.
Normal fibroblasts are characterized by the presence of stress-fibril bundles, the fibrils composed of the complex between actin microfilaments and myosin II. Stress-fibrils terminate by joint adherens complexes (focal contacts), by which, via integrins and many other proteins, the fibroblast is attached to the matrix fibrils. Both stress-fibrils and focal contacts are responsible for the fibroblast stretching on the matrix.
Both in culture in vitro and in connective tissue in vivo different fibroblast variants are present, which seem to transform from one to another. Among these variants, a big fibroblast stands out with a great number of stress-fibrils, many of which contain smooth-muscle actin together with non-muscle actin typical of the fibroblasts [10, 11]. Such fibroblasts are termed myofibroblasts. Other fibroblast variants are smaller both in area and in number of stress-fibrils. Myofibroblasts may play some specific role in connective tissue contraction and vessel constriction. A system of microtubules (tubulin polymers) is not necessary for cell movement, actin filament formation, and actin-myosin fibril contraction. However, this system is necessary for organization of an oriented form of the cell and all its structures, as well as for its directed motion [12].
CHANGES IN FIBROBLAST ORGANIZATION IN CARCINOGENESIS
The well-known morphological features of neoplastic transformation of cultured fibroblasts are weakening of their attachment to the matrix and corresponding diminishing of the cell spread on the substrate, as well as disappearance of actin-myosin stress-fibrils and connected mature integrin-based focal contacts with the substrate [13].
Analysis of one-stage transformation caused by a transfection with the N-ras oncogene has shown that the decrease in area of elongated cells spread on a substrate occurs exclusively due to decrease in width of the transformed cell, whereas its length remains constant [13]. It was shown earlier that the length rather than the width of the fibroblast is determined by an axial system of microtubules [14]. Hence, one can conclude that some structures supporting the lateral spread of the cell undergo changes upon transformation. The chances are that we are dealing with actin-myosin bundles, which, together with focal contacts, form a frame of sorts on the bottom of the cell.
DIFFERENT AND COMMON FEATURES OF THE CYTOSKELETON REORGANIZATION
UPON TRANSFORMATION OF FIBROBLASTS AND EPITHELIUM
Why does EMT, one of the phylo- and ontogenetically ancient normal reorganizations of epithelium, arise regularly during multistage epithelial carcinogenesis? In the course of carcinogenesis, many signaling pathways are activated. In particular, expression of many genes and proteins is induced upon transfection with the Ras oncogene [15, 16]. It would appear reasonable that these reorganizations include those leading to EMT. In other words, it is safe to assume that we deal with the normal signaling pathway, which is activated together with many others upon transformation. EMT in carcinogenesis most likely falls into the same category of phenomena as the synthesis of normal products, such as ectopic hormones and secretions like milk and bile. The normal complete or incomplete EMT most likely passes through selection, because EMT makes the tumor cells or cell groups motile and capable of migration from the tissue. Thus, initially normal EMT can underlie invasion in tumor.
FIBROBLAST TRANSFORMATION AND EMT
Unfortunately, we know very little about phenotypic evolution of fibroblasts in vivo and in vitro. Possibly, the fibroblast “maturation” goes from narrow cells, which lack actin-myosin bundles, to the broad fibroblasts with multiple straight stress-fibrils and then to myofibroblasts, in which the smooth-muscle actin appears in these stress-fibrils. Is this pathway correct? Is this actually the opposite process of “juvenescence”, when broad fibroblasts turn into narrow cells which lack stress-fibrils? Existence of both pathways is possible, but remains to be proved.
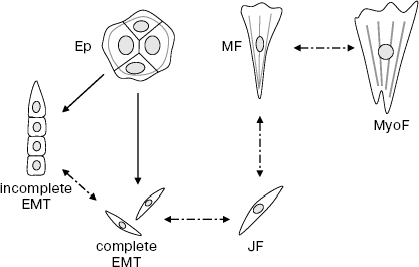
Yet less clear are relations between the “juvenile” “narrow” fibroblasts and narrow mesenchymal cells arising during EMT of epithelium. These two cell types are very homomorphous, and molecular markers distinguishing them are not yet found. So, it is quite a plausible paradoxical supposition that “juvenile” fibroblasts and mesenchymal cells formed during EMT of epithelium are identical. In other words, epithelium and mesenchyme may possess a common predecessor and/or descendant (Scheme).
Scheme of epitheliocyte and fibroblast transitions. Ep, islet of epitheliocytes; JF, juvenile fibroblast; MF, mature fibroblast; MyoF, myofibroblast. Solid arrows, proved transitions; dash-dot arrows, hypothetical transitions
ANALYSIS OF EMT REVEALS PREVIOUSLY UNKNOWN CELL TRANSITION
PATHWAYS IN NORMAL PROCESSES AND IN CARCINOGENESIS
In contemporary biology, “dendroid” schemes of cell transitions in ontogenesis are commonly accepted: from the stem cell to a large set of irreversibly differentiated descendants. A brilliant example of this scheme is histogenesis of hematopoietic and lymphoid cells [17]. Obviously, EMT does not fit this scheme: the initial epithelial cell is not the stem cell, and the final mesenchyme is not a differentiated daughter tissue. When the above stated hypothesis on identity of “juvenile” fibroblasts and mesenchymal descendants in EMT is considered, one is forced to accept that two main histogenetic pathways, namely, geneses of epithelium and mesenchyme, intersect at some common point. It is also possible that these pathways are reversible. Very much is not proved yet, but EMT really exists! It is possible that many alternative cell transitions in Metazoa will be discovered and studied in the near future. There is obvious association of this problem with molecular mechanisms of carcinogenesis.
The author is thankful to E. N. Vasil'eva, S. N. Rubtsova, and V. I. Samoilov for assistance in the manuscript preparation and to D. V. Aiollo, A. Yu. Aleksandrova, M. E. Vaulina, N. A. Glushankova, I. Yu. Zhitnyak, and M. S. Shutova for the data of their experiments.
REFERENCES
1.Ivanov, A. V. (1968) The Origin of Pluricellular
Animals. Phylogenetic Essays [in Russian], Nauka, Leningrad.
2.Vasiliev, J. M., and Gelfand, I. M. (2006)
Biochemistry (Moscow), 71, 821-826.
3.Gherardi, E., and Stoker, M. (1991) Cancer
Cells, 3, 227-232.
4.Thiery, J. P. (2002) Nat. Rev. Cancer,
2, 442-448.
5.De Rooij, J., Kerstens, A., Danuser, G. M.,
Schwartz, M. A., and Waterman-Storer, C. M. (2005) J. Cell
Biol., 171, 153-164.
6.(1905-1915) Selected Papers from the Institute
of Cancer Research: Roy. Canc. Hosp. A. From the Roy. Mersden
Hosp., Vol. 1-10, Lund Humphries, London.
7.Montesano, R., Saint-Vincent, L., Drevon,
C., and Tomatis, L. (1975) Int. J. Canc., 16,
550-558.
8.Bannikov, G. A., Guelstein, V. I.,
Montesano, R., Tint, I. S., Tomatis, L., Troyanovsky, S. M., and
Vasiliev, J. M. (1982) J. Cell Sci., 54, 47-67.
9.Vasiliev, J. M., and Gelfand, I. M. (1981)
Interaction of Normal and Neoplastic Cells with Environment [in
Russian], Nauka, Moscow.
10.Dugina, V., Fontao, L., Chaponnier, C., Vasiliev,
J. M., and Gabbiani, G. (2001) J. Cell Sci., 114,
3285-3296.
11.Dugina, V. B., Aleksandrova, A. Iu., Gabbiani,
G., and Vasil'ev, J. M. (2002) Tsitologiya, 44,
48-55.
12.Vasiliev, J. M., Gelfand, I. M., Domnina, L. V.,
Ivanova, O. Y., Komm, S. G., and Olshewskaja, L. V. (1970) J.
Embryol. Exp. Morphol., 24, 625-640.
13.Kharitonova, M. A., Kopnin, P. B., and Vasiliev,
J. M. (2007) Cell Biol. Int., 31, 220-223.
14.Levina, E. M., Kharitonova, M. A., Rovensky, Y.
A., and Vasiliev, J. M. (2001) J. Cell Sci., 114,
4335-4341.
15.Repasky, G. A., Chenette, E. J., and Der, C. J.
(2004) Trends Cell Biol., 14, 639-647.
16.Shields, J. M., Pruitt, K., McFall, A., Shaub,
A., and Der, C. J. (2000) Trends Cell Biol., 10,
147-154.
17.Chertkov, I. L., and Fridenshtein, A. Ya. (1977)
Cellular Basics of Hemopoiesis [in Russian], Meditsina,
Moscow.