Mitochondria-Targeted Plastoquinone Derivatives as Tools to Interrupt
Execution of the Aging Program.
4. Age-Related Eye Disease. SkQ1
Returns Vision to Blind Animals
V. V. Neroev1, M. M. Archipova1, L. E. Bakeeva2, A. Zh. Fursova3, E. N. Grigorian4, A. Yu. Grishanova3, E. N. Iomdina1, Zh. N. Ivashchenko1, L. A. Katargina1, I. P. Khoroshilova-Maslova1, O. V. Kilina2, N. G. Kolosova3, E. P. Kopenkin5, S. S. Korshunov2, N. A. Kovaleva4, Yu. P. Novikova4, P. P. Philippov2, D. I. Pilipenko2, O. V. Robustova1, V. B. Saprunova2, I. I. Senin2, M. V. Skulachev6, L. F. Sotnikova5, N. A. Stefanova3, N. K. Tikhomirova2, I. V. Tsapenko1, A. I. Shchipanova1, R. A. Zinovkin2, and V. P. Skulachev2,6,7*
1Helmholtz Moscow Research Institute of Eye Diseases, ul. Sadovaya-Chernogryazskaya 14/19, 105062 Moscow, Russia2Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: (495) 939-0338; E-mail: skulach@belozersky.msu.ru
3Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences, pr. Lavrentieva 10, 630090 Novosibirsk, Russia
4Kol'tsov Institute of Developmental Biology, Russian Academy of Sciences, ul. Vavilova 26, 119991 Moscow, Russia
5Skryabin Moscow State Academy of Veterinary Medicine and Biotechnology, ul. Skryabina 23, 109472 Moscow, Russia
6Mitoengineering Center, Lomonosov Moscow State University, 119991 Moscow, Russia
7Faculty of Bioengineering and Bioinformatics, Lomonosov Moscow State University, 119899 Moscow, Russia
* To whom correspondence should be addressed.
Received December 29, 2007; Revision received August 15, 2008
Mitochondria-targeted cationic plastoquinone derivative SkQ1 (10-(6′-plastoquinonyl) decyltriphenylphosphonium) has been investigated as a potential tool for treating a number of ROS-related ocular diseases. In OXYS rats suffering from a ROS-induced progeria, very small amounts of SkQ1 (50 nmol/kg per day) added to food were found to prevent development of age-induced cataract and retinopathies of the eye, lipid peroxidation and protein carbonylation in skeletal muscles, as well as a decrease in bone mineralization. Instillation of drops of 250 nM SkQ1 reversed cataract and retinopathies in 3-12-month-old (but not in 24-month-old) OXYS rats. In rabbits, experimental uveitis and glaucoma were induced by immunization with arrestin and injections of hydroxypropyl methyl cellulose to the eye anterior sector, respectively. Uveitis was found to be prevented or reversed by instillation of 250 nM SkQ1 drops (four drops per day). Development of glaucoma was retarded by drops of 5 µM SkQ1 (one drop daily). SkQ1 was tested in veterinarian practice. A totally of 271 animals (dogs, cats, and horses) suffering from retinopathies, uveitis, conjunctivitis, and cornea diseases were treated with drops of 250 nM SkQ1. In 242 cases, positive therapeutic effect was obvious. Among animals suffering from retinopathies, 89 were blind. In 67 cases, vision returned after SkQ1 treatment. In ex vivo studies of cultivated posterior retina sector, it was found that 20 nM SkQ1 strongly decreased macrophagal transformation of the retinal pigmented epithelial cells, an effect which might explain some of the above SkQ1 activities. It is concluded that low concentrations of SkQ1 are promising in treating retinopathies, cataract, uveitis, glaucoma, and some other ocular diseases.
KEY WORDS: mitochondria, antioxidants, retinopathies, cataract, uveitis, glaucomaDOI: 10.1134/0006297908120043
Abbreviations: MDA, malondialdehyde; ROS, reactive oxygen species; RPE, retinal pigmented epithelium; SkQs, cationic derivatives of plastoquinone or methyl plastoquinone; SkQ1, 10-(6′-plastoquinonyl) decyltriphenylphosphonium.
As reported in the preceding papers [1-3], very small amounts of mitochondria-targeted
plastoquinone derivatives (SkQs) show pronounced antioxidant effects on
model membranes, isolated mitochondria, and cell cultures. In the
latter case, strong antiapoptotic and antinecrotic effects were
observed in situations when cell death was induced or mediated by
reactive oxygen species (ROS) [1]. It was also
found that several age-related pathologies (arrhythmia, heart and
kidney infarctions, stroke, p53-controlled lymphomas, and certain other
types of cancer) can be successfully treated by SkQs in animals [2, 3].
In this paper, we shall continue the latter approach when studying age-related, ROS-mediated ocular diseases. In fact, retina is a tissue of the the highest risk of the ROS-induced damage since (i) it contains high level of polyunsaturated fatty acids, a very good target for ROS, (ii) it is exposed to light producing such ROS as singlet oxygen, and (iii) oxygen concentration in retina is near-arterial, i.e. much higher than in the great majority of other tissues (in spite of the fact that retina is a tissue of highest respiratory activity) [4].
There are numerous indications of a crucial role of ROS in the main age-related ocular pathologies, i.e. retinopathies (maculodystrophy [5, 6], retinitis pigmentosa [7, 8], hereditary optic neuropathy [9]), as well as glaucoma [10, 11], cataract [12, 13], and autoimmune uveitis [14, 15]. Polyunsaturated fatty acids in mitochondrial cardiolipin are first of all attacked by mitochondria-produced ROS that are quenched by SkQs [1]. This is why the above-listed pathologies attracted our attention as a possible field of therapeutic application of SkQs. Here we shall describe certain results obtained when animals suffering from retinopathies, cataract, uveitis, glaucoma, and some other eye diseases were treated with SkQ.
MATERIALS AND METHODS
Studies with OXYS rats. Lipid peroxidation and protein carbonylation in skeletal muscles of OXYS and Wistar rats were investigated as described in refs. [16] and [17], respectively.
Mineralization of vertebra and bones of extremities were measured with a LUNAR Expert-XL X-ray bone densitometer.
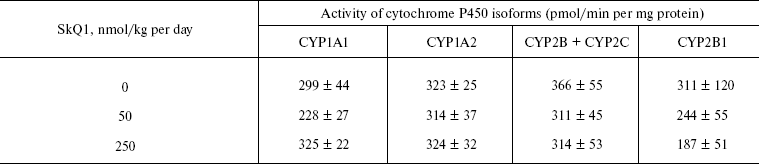
Measurements of activity of cytochrome P450 isoforms. Microsomes were isolated from OXYS rat livers perfused by solution of 1.15% KCl and 20 mM Tris-HCl, pH 7.4. The perfused liver was homogenized in solution of the same composition. Supernatant obtained after low-speed centrifugation of homogenate was centrifuged at 100,000g for 1 h. Sediment of microsomes was suspended in 0.1 M potassium phosphate, pH 7.4, supplemented with 20% glycerol.
Levels of the P450 isoforms CYP1A1, CYP1A2, CYP2B + CYP2C, and CYP2B1 were fluorometrically measured as a rate of formation of resorufin from 7-ethoxyresorufin, 7-methoxyresorufin, 7-benzoxyresorufin, and 7-penthoxyresorufin, respectively [18].
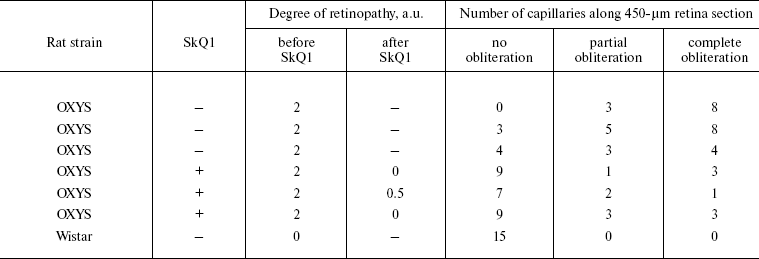
Ophthalmoscopic studies of OXYS and Wistar rats were carried out with a Betta ophthalmoscope, an Opton fundus-camera, and a Shin-Nippon SL-45 slit lamp. Degree of cataract was estimated as follows: 1 arbitrary unit (a.u.), certain lens area of slightly decreased transparency; 2 a.u., certain area of strongly decreased transparency; 3 a.u., strong decrease in transparency of the whole lens cortex and/or nucleus.
As to retinopathy, also three major stages of retinal damage were distinguished, namely, 1 a.u., appearance of drusen and other pathological changes in the retinal pigmented epithelium (RPE) and a partial atrophy of a choroid capillary layer; 2 a.u., exudative detachment of RPE and of retinal neuroepithelium, further choroid capillary layer atrophy; 3 a.u., neovascularization and exudative-hemorrhagic detachment of RPE and neuroepithelium; scarring.
Studies of experimental uveitis. Arrestin purification. Bovine retinal outer segments were prepared from retinas following Wilden and Kuhn [19]. Arrestin was purified by specific binding of this protein to phosphorylated and photoactivated rhodopsin [20], followed by chromatographic separations on a DEAE-cellulose column and on a Mono Q column [21].
Immunization of rabbits with arrestin. Arrestin solution (1 ml in PBS, 0.8 mg/ml) was emulsified with equal volume of Freund's complete adjuvant (Sigma, USA) and inoculated into 6-month-old New Zealand white rabbits. The second inoculation was performed on day 30. Animals treated with Freund's adjuvant served as a control. After immunization, blood samples were taken from the rabbits and production of antibody was measured using an ELISA test.
Measurement of nitrate level in the aqueous humor of anterior sector. Nitric oxide was determined using a spectrophotometric assay based on the Griess reaction. Briefly, 100 µl samples of the aqueous humor of the eye anterior sector were mixed with 1% sulfanilamide and 0.1% naphthyl ethylenediamine. After 10 min incubation at room temperature, we determined the concentration of nitrate in the mixture by measuring absorbance at 550 nm and comparing this value with absorbance of standard solutions of sodium nitrate.
SkQ treatment. SkQ1 was dissolved in a solution containing 10 mM potassium phosphate (pH 6.5) and 0.9% NaCl. Drops of the SkQ1 solution were instillated to one of rabbit's eyes (four times per day).
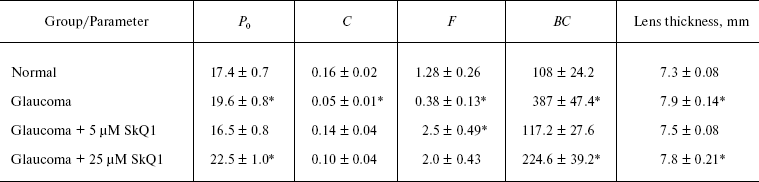
Studies of experimental glaucoma. Experimental glaucoma was induced by 10 injections (two injections per week) of 0.1 ml viscoelastic Celoftal (2% hydroxypropyl methyl cellulose) to anterior sector of the both eyes of a rabbit [22]. To the right eye, Vetomitin, a pharmaceutical form of SkQ1, was daily instilled whereas the left eye was used as a control to the SkQ1 treatment.
Among rabbits treated with SkQ1, four animals obtained drops containing 5 µM SkQ1. Other four rabbits obtained 25 µM SkQ1. Before the first Celoftal injection, the eyes were investigated to measure parameters of the healthy animals. The following devices were used: a GlauTest-60 eye tonograph, an Angiodin-Ophthalmo (Russia) ultrasonic ophthalmoscope, and a Cannon (Japan) fundus-camera.
Cultivation of rat eye posterior sector. Preparations of eye posterior sector were obtained from eyes of narcotized adult (2-3 months) Wistar rats (the treatment carried out according to bioethics regulations of the Russian Academy of Sciences). Procedures of obtaining, roller cultivation, fixation, as well as methods of investigation of the preparations are described in detail in [23]. Briefly, the eye anterior sector containing the cornea, iris, and lens was removed and two types of the remaining posterior sector were used for roller cultivation. In one case, the posterior eye sector before cultivation was incubated for 3-5 times in 5 ml commercial DMEM medium with phenol red, 3% L-glutamine, 4% gentamicin (medium A), and 10 mM EDTA. Afterward, neural retina was removed and the remaining part of the sector (i.e. RPE, choroid, and sclera) was used. In the other case, neural retina was not removed. In both cases, samples of the eye posterior sector were put into flasks containing medium A and 10% fetal calf serum with 20 nM SkQ1 or without SkQ1. Then the flasks were placed into a roller and cultivated in a dark sterile box at 60 rpm and 35.5°C for 7 or 14 days. The medium was not changed during the whole period of roller cultivation. After cultivation, the samples were fixed with 4% formaldehyde and Bouin's solution. Serial 7-µm cross-sections (Reichert OME microtome) were stained with hematoxylin and eosin. To visualize macrophages, an anti-macrophage antibody (Sigma) was applied. Images obtained were scrutinized using an Olympus AH-3 microscope, a digital camera, and a computer with Lite, Corel Draw, Adobe Photoshop, Excel, and Plot Calc program packages.
RESULTS
Prevention and reversal of age-dependent cataract and retinopathy in OXYS rats. As a model for in vivo antioxidant effects of SkQs, we used OXYS rats, a strain suffering from constant oxidative stress [24]. In these rats, cataract and retinopathy were shown to appear as early as at three-month age [25-27].
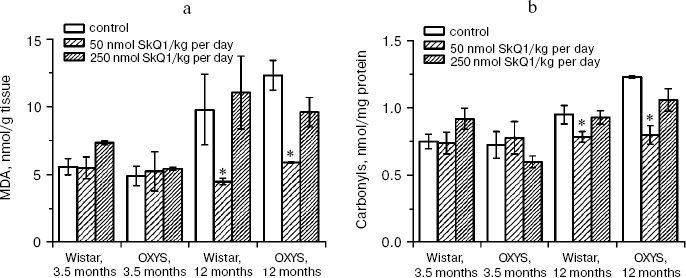
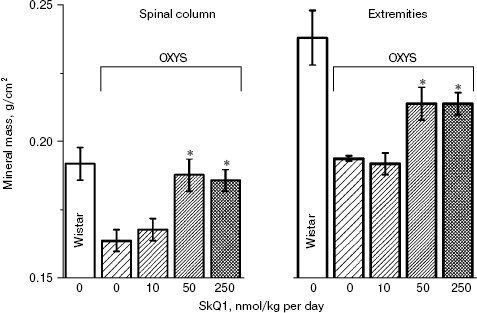
In the first series of experiments, we investigated age-dependent consequences of oxidative stress in OXYS rats, trying to abrogate them by means of SkQ1 added to their food. It was found (Fig. 1, a and b) that levels of lipid peroxidation (estimated by measuring malondialdehyde (MDA)) and oxidation (carbonylation) of proteins are higher in skeletal muscles of one-year-old Wistar rats than in three-month-old ones. The effect of age was even larger in OXYS rats. Feeding with very small amount of SkQ1 (50 nmol/kg per day) resulted in a decrease in the lipid and protein oxidation levels. As to the mineral mass levels in vertebra and extremities, these parameters were lower in OXYS than in Wistar rats due to progeria-induced osteoporosis. Again, SkQ1 feeding proved to be favorable, increasing the mineral mass in OXYS rats (Fig. 2).
Fig. 1. Age-dependent increase in lipid peroxidation (a) and protein carbonylation (b) in skeletal muscles of OXYS and Wistar rats is abolished by adding to the food 50 nmol SkQ1/kg per day. Here and below: vertical bars, standard errors; *, probability of insignificant difference between the SkQ1-treated and SkQ1-nontreated groups p < 0.05.
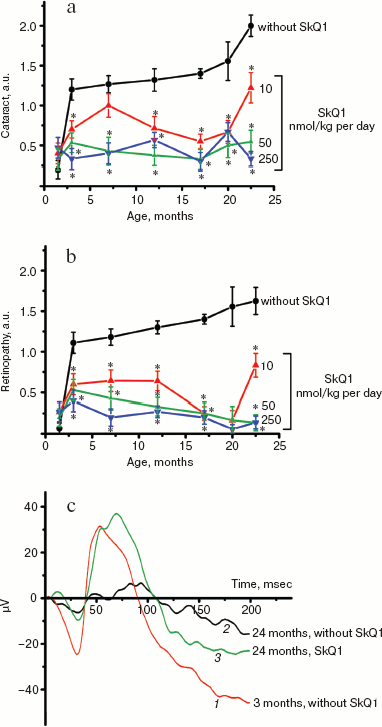
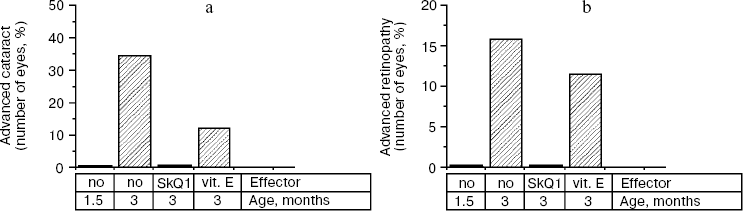
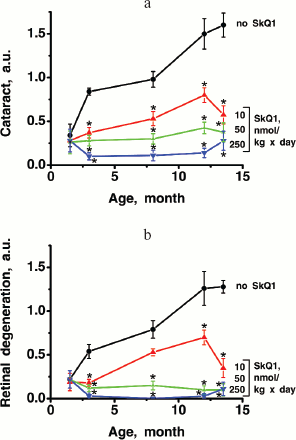
Thus, the presented data show that SkQ1 is competent in preventing some consequences of oxidative stress in OXYS rats. Then an attempt was made to treat by SkQ1 eye diseases in OXYS rats. As experiments showed, addition of the same SkQ1 amounts to the food completely prevented development of cataract and retinopathy in OXYS rats up to age of two years (Fig. 3 (see color insert) and Table 1; see also Supplementary information, Figs. S1 and S21). Vitamin E added to the food was much less effective than SkQ1 (Fig. 4). It is remarkable that the SkQ1 effects were not accompanied by any induction of cytochromes P450 in liver (Table 2), in contrast to those of vitamin E (not shown).Fig. 2. SkQ1 prevents development of osteoporosis in OXYS rats. Ordinate, mineral mass of corresponding bones. Age of rats, 9.5 months. SkQ1 was added to the food at age 1.5 months. * p < 0.05.
1 Supplementary Information is linked to the online version of the paper.
Table 1. Effect of SkQ1 added to food (250 nmol/kg per day) on the b-wave magnitude of the rat electroretinogramFig. 3. SkQ1 prevents development of cataract (a) and retinopathies (b) in OXYS rats. c) Electroretinograms of three rats: 1) 3 months, no SkQ1; 2) 24 months, no SkQ1; 3) 24 months, 250 nmol SkQ1/kg per day with food. Flash, 3 cd⋅sec/m2.
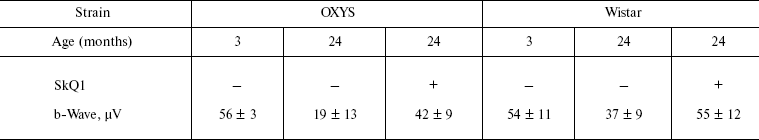
Note: Standard errors are indicated (each group contained 5-12 animals).
Table 2. SkQ1 feeding for 1.5 months does not affect levels of cytochrome P450 in liver microsomes of 14.5-month-old OXYS ratsFig. 4. Comparison of protective effects of SkQ1 (50 nmol/kg per day) and vitamin E (500 µmol/kg per day) against cataract (a) and retinopathies (b) in OXYS rats.
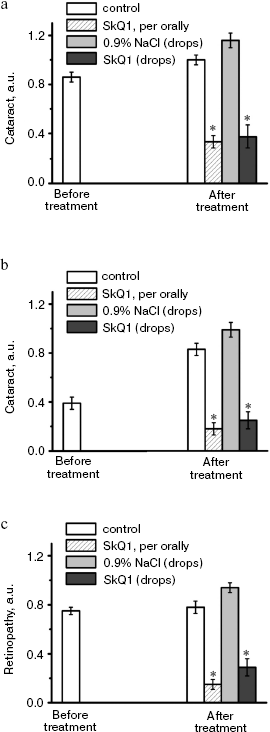
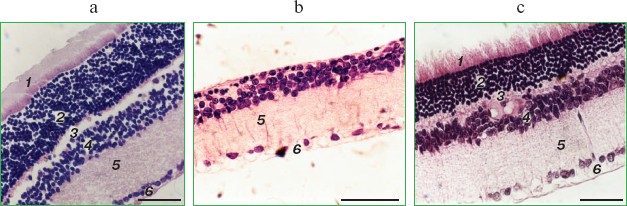
It was also found that instillations of drops of nanomolar SkQ1 significantly reverse pathological changes in middle-age OXYS animals (Figs. 5 and 6). The latter effect was also observed in Wistar rats suffering from cataract (Figs. 5 and 6). In very old (more than 18 months) rats, neither cataract nor retinopathy was reversed by SkQ1 (not shown), although it still effectively prevented the diseases (Fig. 3, a-c, see color insert). The above conclusion concerning preventive effect of SkQ1 on 24-month-old OXYS rats was confirmed by histological analysis of sections across the retina (Fig. 7; see color insert). The figure shows that in OXYS rats without SkQ1 the photoreceptor layer is absent, whereas OXYS rats receiving SkQ1 during all their life retained this layer. In old Wistar rats, the photoreceptor layer was present even without SkQ1. These results are in line with our observations that the electroretinogram disappeared in the majority of the 24-month-old OXYS rats but was retained in OXYS rats with SkQ1 as well as in Wistar rats (Fig. 3c and Table 1).
Fig. 5. Instillations of the 250 nM SkQ1 drops or feeding of 50 nmol SkQ1/kg per day during 1.5 months reverse already developed cataract and retinopathies in OXYS (a, c) and Wistar (b) rats. Age before treatment, 9 months. * p < 0.05.
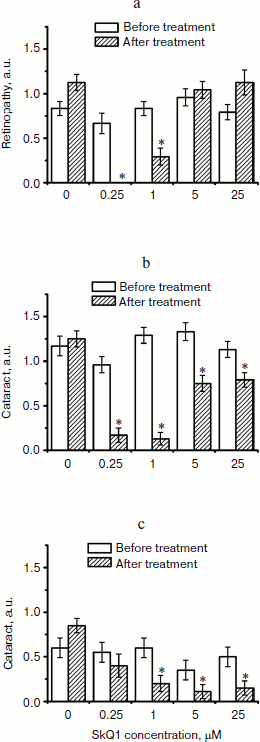
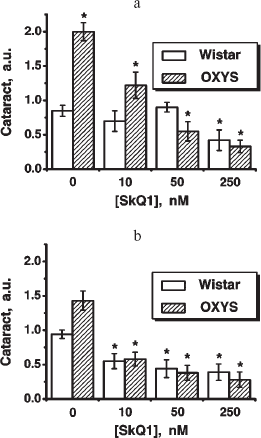
Fig. 6. Therapeutic effect of various SkQ1 concentrations upon already developed retinopathies and cataract in OXYS (a, b) and Wistar (c) rats. The treatment (one drop of SkQ1 solution daily) was started when rats were 9-month-old. Drops of SkQ1 were instilled during 52 days. In each group, 24 eyes of 12 animals (a, b) or 20 eyes of 10 animals (c) were studied. * p < 0.05.
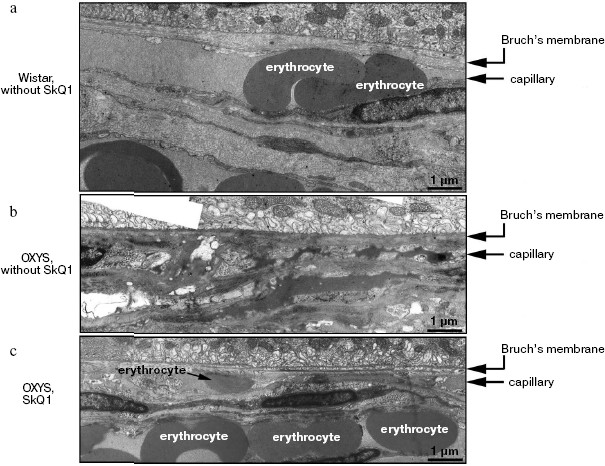
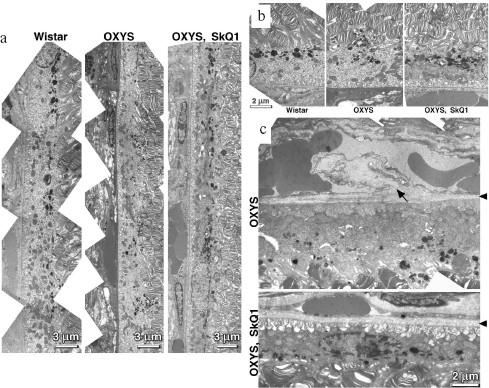
Reversal of an already developed retinopathy by SkQ1 drops was confirmed by electron microscopy data. As shown in Fig. 8 and Table 3, retinopathy in 11-month-old OXYS rats results in obliteration of choriocapillaries. This parameter is at least partially normalized after 1.5-month instillations of 250 nM SkQ1 (one drop daily). Reappearance of choriocapillaries in the presence of SkQ1 was accompanied with normalization of some other morphological features, i.e. distribution of lipofuscin granules in retinal pigment epithelial cells (Fig. S3, a and b) and disappearance of hernias formed due to disruption of Bruch's membrane (Fig. S3c).Fig. 7. A histological study of sections across the retinas of 24-month-old Wistar (a) and OXYS (b, c) rats. Staining with hematoxylin and eosin. a) Wistar rats: the normal retina structure is well seen, i.e. (1) rods, (2) outer nuclear layer, (3) outer plexiform layer, (4) inner nuclear layer, (5) inner plexiform layer, and (6) ganglionic cell layer. b) OXYS without SkQ1: there is no photoreceptor compartment (rods as well as outer nuclear and plexiform layer are absent). c) OXYS rats obtained SkQ1 with food from age 1.5 month (250 nmol/kg per day). All the layers are present. Typical results are shown. Sixteen eyes (8 rats) were studied in each group. Bar, 50 µm.
Table 3. State of choriocapillaries in retina of 11-month-old rats. Therapeutic effect of SkQ1 (one drop of 250 nM SkQ1 per day during the last 68 days)Fig. 8. Electron microscopic study of retina of 11-month-old Wistar (a) and OXYS (b, c) rats. c) Drops of 250 nM SkQ1 were instilled during the last 68 days
Favorable effects of SkQ1 can disappear when it was added in excess. In skeletal muscles of OXYS rats, this occurred at 250 nmol SkQ1/kg per day (Fig. 1). In bones of the same rats, 250 nmol SkQ1 was still as effective as 50 nmol (Fig. 2). In eyes of OXYS rats, drops of 10 nM-1 µM SkQ1 were effective in reversal of cataract and retinopathy, 5 µM being ineffective. As to eyes of Wistar rats, even 25 µM SkQ1 was still of favorable activity (Figs. 5 and 6). The above relationships can be explained assuming that (i) disappearance of therapeutic action of SkQ1 is due to prooxidant activity of its high concentrations [1] in OXYS rats suffering from constant oxidative stress.
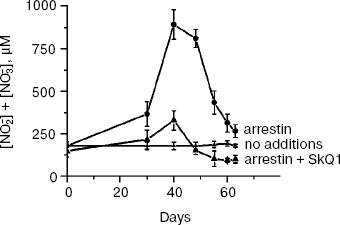
SkQ1 prevents and reverses blindness in experimental uveitis. Uveitis has also been shown to be mediated by mitochondrial ROS [25], so we tried to use SkQ1 for treatment of this eye disease, too. To this end, a rabbit model of experimental uveitis was investigated. Experimental uveitis was induced by immunization of the animal by a photoreceptor-specific protein, arrestin, which resulted in blindness. This effect was prevented and reversed by SkQ1 instillations (four drops of 250 nM SkQ1 per day; not shown). It was also found (Fig. 9) that the same SkQ1 treatment strongly inhibited formation of NO2- and NO3- in eyes of the uveitis-suffering animals (under uveitis, these processes are known to be initiated by interaction of NO with O2· [28, 29]).
SkQ1 prevents development of experimental glaucoma in rabbits. In rabbits, experimental glaucoma was induced by a series of Celoftal instillations to the eye anterior sector. This resulted in appearance of such typical glaucoma features as an increase in intraocular pressure (P0), a strong decrease in the aqueous humor outflow (C) as well as in humor production (F), a strong rise of Bekker's coefficient (BC), and some increase of the lens thickness (Table 4). Analysis of photographs obtained using a fundus camera revealed excavation of the optic disk (not shown). All these parameters were normalized if treatment with the glaucoma inducer was accompanied by instillation of drops of 5 µM SkQ1 solution. Higher (25 µM) (Table 4) and lower (0.25 µM; not shown) SkQ1 concentrations proved to be less efficient than 5 µM SkQ1.Fig. 9. SkQ1 prevents experimental uveitis-induced increase in nitrite and nitrate levels in the aqueous humor of the eye anterior sector. Twenty eyes (10 rabbits) were studied, namely, six eyes without and 14 eyes with SkQ1 (four drops of 250 nM SkQ1 were instilled daily during 33 days).
Table 4. Drops of 5 µM SkQ1
prevent development of experimental glaucoma in rabbits
*p < 0.05 for the eye with experimental glaucoma
vs. normal eye.
SkQ1 prevents macrophagal transformation of RPE and destruction of retina ex vivo. A number of retinopathies are known to be accompanied with a ROS-mediated transformation of the retinal pigment epithelial (RPE) cells to macrophages that attack other retinal cells [29, 30]. If this process is arrested by SkQ1, this might explain, at least partially, the above described favorable effect of our compound upon certain retinopathies and uveitis.
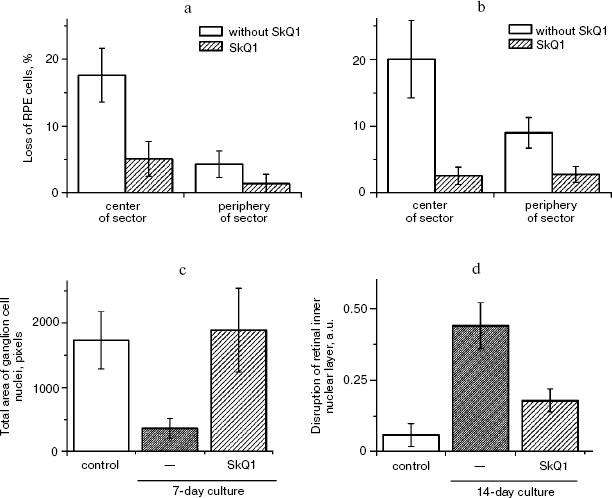
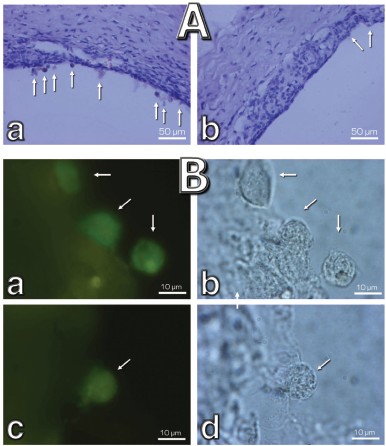
Macrophagal RPE cell transformation was studied in an ex vivo system, i.e. eye posterior sector obtained from two-month-old Wistar rats and cultivation in a sterile box for 7 (Fig. 10, a and c) or 14 (Fig. 10, b and d) days. As one can see in Fig. 10a, one week cultivation results in some loss of RPE cells, which was more pronounced in the center of the sector. Simultaneously, the number of macrophages was strongly increased (Fig. S4). Both these effects were strongly inhibited by 20 nM SkQ1 added to the cultivation medium. This action paralleled prevention of disappearance of the ganglion cells (Fig. 10c) and of inner nuclear layer of the photoreceptor cells (Fig. 10d).
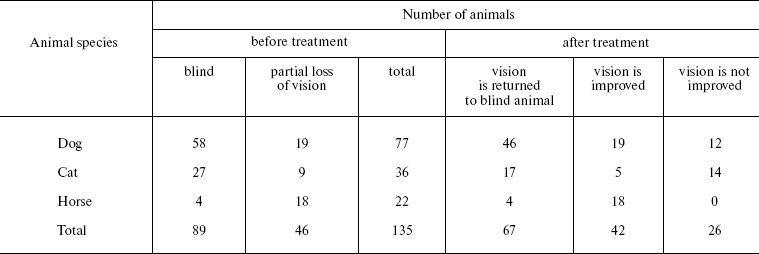
Veterinary practice experiment of application of SkQ1 to treat various animal ocular pathologies. Finally, SkQ1 was applied in veterinarian practice in cases when conventional medical treatments failed. A total of 135 animals (dogs, cats, and horses) suffering from various retinopathies were treated daily with drops of 250 nM SkQ1. In 89 cases, the animals were completely blind before the treatment. Vision was returned to 67 of them (Table 5). There was not a single case when SkQ1 had an unfavorable effect or its efficiency declined in the course of the treatment time.Fig. 10. Protective effect of 20 nM SkQ1 on the posterior sector of the rat eye during 7 (a, c) or 14 days (b, d) of roller cultivation. The center and peripheral regions of the sector were analyzed. a, b) After cultivation. c, d) White columns, before cultivation; black or striped columns, after cultivation without or with SkQ1, respectively.
Table 5. Therapeutic effect of instillations
of Vetomitin (drops of 250 nM SkQ1) on retinopathies
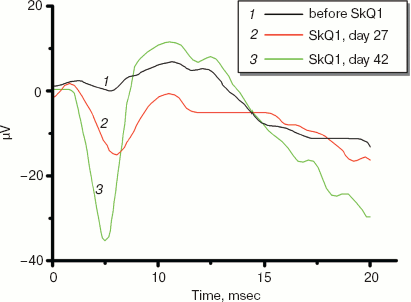
Electroretinograms of a dog whose visual function was recovered by means of 250 nM SkQ1 instillations are shown in Fig. 11 (see color insert). The dog was blind because of inherited retinal dysplasia. As the figure shows, before the SkQ1 treatment there was practically no electric response to light. After 27 days of SkQ1 instillations, visual function was partially recovered and some electric response appeared. Even larger response was revealed on day 42 of the treatment, which was accompanied with further improvement of vision (for details, see Supplementary Information).
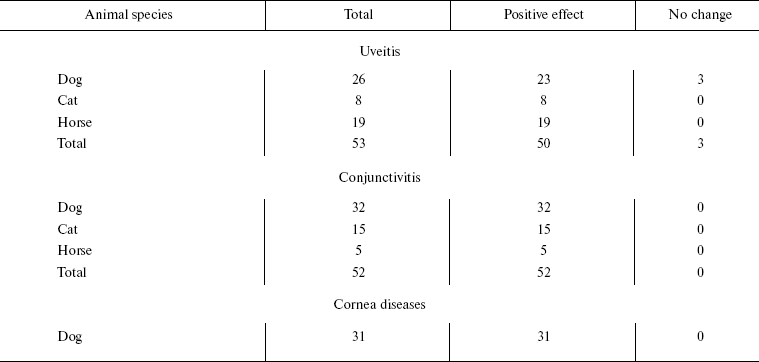
Among dogs, cats, and horses suffering from retinopathies were those with inherited retinal dysplasia (degeneration), progressing retinal degeneration, or a secondary retinal degeneration. The best results of the SkQ1 treatment were obtained with inherited dysplasia (a positive effect in 67% cases) and secondary degeneration (in 54% cases). As to progressing degeneration, SkQ1 helped in 29% cases. Moreover, SkQ1 was effective in some cases of dry eye syndrome as well as for treatment of uveitis and some other autoimmune eye diseases, conjunctivitis, and certain corneal diseases (Table 6). SkQ1 drops were without effect on neuro-ophthalmological pathologies.Fig. 11. Electroretinograms of the left eye of dog Greta (for description of the case, see Supplementary information). Where indicated, one drop of 250 nM SkQ1 was daily applied for 27 or 42 days.
Table 6. Therapeutic effect of instillation
of Vetomitin (drops of 250 nM SkQ1) on uveitis, conjunctivitis,
and corneal diseases
DISCUSSION
As mentioned above, retina is a tissue where risk of ROS-induced damage is highest. The O2 concentration in retina is almost as high as in lung, which increases probability of ROS formation. In addition, in contrast to lung, retina is illuminated by the light also initiating generation of ROS. Moreover, the amount of polyunsaturated lipids (which are targets for ROS) is much higher in retina than in lung and other tissues [4]. It is not surprising, therefore, that the most frequent ocular diseases are ROS-mediated [4-15].
Mitochondria are usually either the primary source of ROS [31, 32] or mediators of burst of ROS formation in other cellular compartments (“ROS-induced ROS release” [1, 33]). This is why it was reasonable to try mitochondria-targeted rechargeable antioxidant SkQ1 as a medicine to treat ocular diseases. OXYS rats suffering from constant oxidative stress proved to be a convenient model for such a study. Here it was found that small amounts of SkQ1, i.e. 50 nmol (or 0.03 mg)/kg per day, decreased levels of lipid peroxidation and protein carbonylation in skeletal muscles, and increased mineralization of bones of OXYS rats, which was decreased due to osteoporosis (Figs. 1 and 2). These data indicated that SkQ1 could mitigate oxidative stress and related progeria in OXYS rats.
Further experiments showed that the same amounts of SkQ1 prevented development of cataract and retinopathies, which appeared in the control (SkQ1 non-treated) OXYS rats as early as at 3rd month of life. With SkQ1 (50 nmol/kg per day), these age-induced diseases did not develop at least up to 2-years of age (Figs. 3 (a and b), 7, and S1 (a and b)). At this age, the majority of OXYS rats were practically blind, showing no light-induced electric response of the retina. The response was retained if animals were fed with SkQ1 (Fig. 3c and Table 1). Vitamin E proved to be much less efficient that SkQ1. Even 500 µmol vitamin E/kg per day (i.e. 10,000-fold higher than SkQ1 dose) decreased the cataract and retinopathy levels far less strongly than SkQ1 (Fig. 4). Importantly, the SkQ1 amounts competent in successful treating ocular diseases were without effect upon the cytochrome P450 levels in liver endoplasmic reticulum (Table 2), in contrast to vitamin E which induced these cytochromes. Such a difference between SkQ1 and vitamin E could be explained by (i) much lower amount of SkQ1 used and (ii) the fact that mitochondria should win over other intracellular compartments (including endoplasmic reticulum where cytochrome P450 is localized) in competition for SkQ.
If a rat was not too old, daily instillations of one drop of extremely diluted (250 nM) SkQ1 solution were found to be of excellent therapeutic effect upon already developed cataract and retinopathies (Figs. 5, 6, and 8). In this case, the amount of SkQ1 added daily was as small as about 0.015 µg/kg per day (cf. 0.03 mg/kg per day in the experiments where SkQ1 was received with the food).
Obliteration of choriocapillaries is a typical feature of retinopathy development in OXYS rats. In 11-month-old animals, this pathology was effectively reversed by SkQ1 drops as seen in electron micrographs shown in Fig. 8. It should be mentioned that an attempt to treat inherited retinopathies by a mitochondria-targeted antioxidant was recently undertaken by Wright and coworkers [34]. They used MitoQ and failed to obtain any positive result. The reason for this might be small size of the window between anti- and prooxidant concentrations of MitoQ [1].
Retinopathies in both OXYS rats and Wright's mice were a consequence of certain genetic injuries. We tried to use our compound to treat two experimentally induced eye pathologies, i.e. uveitis and glaucoma.
In rabbits, experimental uveitis was induced by immunization of the animals with the retinal protein arrestin, which resulted in blindness. Vision was returned by a 5-week course of instillations of four drops of 250 nM SkQ1 per day. Pretreatment with the SkQ1 drops prevented development of uveitis, which was seen not only in photographs of a fundus camera but also when the NO2- + NO3- level in the aqueous humor of eye anterior sector was measured (Fig. 9). The uveitis experiments were especially demonstrative since the SkQ1 drops were instilled into one eye only, whereas the other eye remained non-treated as a control. As a result, all the rabbits in the cages turned in a way allowing viewing by the treated eye of a person coming into the room.
Instillations of 5 µM SkQ1 were very effective in preventing experimental glaucoma induced by injection of Celoftal to the eye anterior sector of rabbits (Table 4). Like some other measured parameters, the efficiency of SkQ1 showed a concentration optimum. The efficiency lowered when SkQ1 concentration increased from 5 to 25 µM or decreased to 0.25 µM.
The medicine Vetomitin containing 250 nM SkQ1 was tested at the Department of Ophthalmology, K. I. Skryabin Russian Veterinarian Academy. Almost 300 dogs, cats, and horses suffering from retinopathies, uveitis, conjunctivitis, and cornea diseases were treated by instillations of Vetomitin drops. In more than 200 cases, obvious improvement was observed. Treatment of animals becoming blind due to retinopathies was demonstrative. Vision returned to 67 of 89 our animal patients (Table 5). For certain case descriptions, see Supplementary Information.
In an ex vivo study, the mechanism of SkQ1 effects was investigated. In these experiments, it was found that transformation of RPE cells to macrophages [29, 30] in cultivated eye posterior sector is strongly decreased by SkQ1 at concentration as low as 20 nM (Fig. 10). Such an inhibition of this ROS-linked process might at least partially explain the favorable effect of SkQ1 on retina in various ocular pathologies.
We are very grateful Professor V. A. Sadovnichii, Rector of Moscow State University, for his interest in the project and encouragement. We thank E. Yu. Zernyy and V. A. Churyumov for their participation in certain experiments. Generous support of Mr. O. V. Deripaska, which in fact made possible this study, is greatly appreciated.
Supported by Mitotechnology LLC, M. V. Lomonosov Moscow State University, the Vol'noe Delo Foundation (grant No. 99F-06), Russian Ministry of Education and Science (grant “Leading Scientific Schools” No. 5762.2008.4).
REFERENCES
1.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E.,
Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Yu,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletjushkina, O. Yu., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Yu., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P.
(2008) Biochemistry (Moscow), 73, 1273-1287.
2.Bakeeva, L. E., Barskov, I. V., Egorov, M. V.,
Isaev, N. K., Kapelko, V. I., Kazachenko, A. V., Kirpatovsky, V. I.,
Kozlovsky, S. V., Lakomkin, V. L., Levina, S. B., Pisarenko, O. I.,
Plotnikov, E. Y., Saprunova, V. B., Serebryakova, L. I., Skulachev, M.
V., Stelmashook, E. V., Studneva, I. M., Tskitishvili, O. V., Vasileva,
A. K., Victorov, I. V., Zorov, D. B., and Skulachev, V. P. (2008)
Biochemistry (Moscow), 73, 1288-1299.
3.Agapova, L. S., Chernyak, B. V., Domnina, L. V.,
Dugina, V. B., Efimenko, A. Yu., Fetisova, E. K., Ivanova, O. Yu.,
Kalinina, N. I., Khromova, N. V., Kopnin, B. P., Kopnin, P. B.,
Korotetskaya, M. V., Lichinitser, M. R., Lukashev, A. L., Pletjushkina,
O. Yu., Popova, E. N., Skulachev, M. V., Shagieva, G. S., Stepanova, E.
V., Titova, E. V., Tkachuk, V. A., Vasiliev, J. M., and Skulachev, V.
P. (2008) Biochemistry (Moscow), 73, 1300-1316.
4.Kanda, A., Chen, W., Othman, M., Branham, K.
E., Brooks, M., Khanna, R., He, S., Lyons, R., Abecasis, G. R.,
and Swaroop, A. (2007) Proc. Natl. Acad. Sci. USA, 104,
16227-16232.
5.Justilien, V., Pang, J. J., Renganathan, K., Zhan,
X., Crabb, J. W., Kim, S. R., Sparrow, J. R., Hauswirth, W. W., and
Lewin, A. S. (2007) Invest. Ophthalmol. Vis. Sci., 48,
4407-4420.
6.King, A., Gottlieb, E., Brooks, D. G., Murphy, M.
P., and Dunaief, J. L. (2004) Photochem. Photobiol., 79,
470-475.
7.Komeima, K., Rogers, B. S., Lu, L., and
Campochiaro, P. A. (2006) Proc. Natl. Acad. Sci. USA,
103, 11300-11305.
8.Komeima, K., Rogers, B. S., Lu, L., and
Campochiaro, P. A. (2007) J. Cell Physiol., 213,
809-815.
9.Ghelli, A., Porcelli, A. M., Zanna, C., Martinuzzi,
A., Carelli, V., and Rugolo, M. (2008) Invest. Ophthalmol. Vis.
Sci., 49, 671-676.
10.McKinnon, S. J. (2003) Front. Biosci.,
8, 1140-1156.
11.Tezel, G. (2006) Prog. Retin. Eye Res.,
26, 490-513.
12.Olofsson, E. M., Marklund, S. L., and Behndig, A.
(2007) Free Rad. Biol. Med., 42, 1098-1105.
13.Lassen, N., Bateman, J. B., Estey, T., Kuszak, J.
R., Nees, D. W., Piatigorsky, J., Duester, G., Day, B. J., Huang, J.,
Hines, L. M., and Vasiliou, V. (2007) J. Biol. Chem.,
282, 25668-25676.
14.Brito, B. E., Marcano, J. C., Salazar, E.,
Cano, M., Baute, L., Bernal, G., and Gonzalez, L. R. (2006)
Ocul. Immunol. Inflamm., 14, 117-124.
15.Pararajasegaram, G., Sevanian, A., and Rao, N. A.
(1991) Ophthalm. Res., 23, 121-127.
16.Kovachich, G. B., and Mishra, O. P. (1980) J.
Neurochem., 35, 1449-1452.
17.Reznick, A., and Packer, L. (1994) Meth.
Enzymol., 233, 357-363.
18.Burke, M. D., Thompson, S., Weaver, R. J., Wolf,
C. R., and Mayer, R. T. (1994) Biochem. Pharmacol., 48,
923-936.
19.Wilden, U., and Kuhn, H. (1982)
Biochemistry, 21, 3014-3022.
20.Senin, I. I., Zargarov, A. A., Alekseev, A. M.,
Gorodovikova, E. N., Lipkin, V. M., and Philippov, P. P. (1995) FEBS
Lett., 376, 87-90.
21.Palczewski, K., Pulvermuller, A., Buczylko, J.,
and Hofmann, K. P. (1991) J. Biol. Chem., 266,
18649-18654.
22.Moreno, M. C., Campanelli, J., Sande, P., Saenz,
D. A., Sarmiento, M. I. K., and Rosenstein, R. S. (2004) Free Rad.
Biol. Med., 37, 803-813.
23.Grigoryan, E. N., Novikova, Yu. P., Kilina,
O. V., and Philippov, P. P. (2007) Byul. Eksp. Biol. Med.,
144, 618-625.
24.Solovyeva, N. A., Morozkova, N. C., and Salganik,
R. I. (1975) Genetika, 11, 63-71.
25.Kolosova, N. G., Lebedev, P. A., Aidagulova, S.
V., and Morozkova, T. S. (2003) Byul. Eksp. Biol. Med.,
136, 415-419.
26.Sergeeva, S., Bagryanskaya, E., Korbolina, E.,
and Kolosova, N. (2006) Exp. Gerontol., 41, 141-150.
27.Kolosova, N. G., Shcheglova, T. V., Sergeeva, S.
V., and Loskutova, L. V. (2006) Neurobiol. Aging,
27, 1289-1297.
28.Rajendram, R., Saraswathy, S., and Rao, N. A.
(2007) Br. J. Ophthalmol., 91, 531-537.
29.Machemer, R., and Laqua, H. (1975) Am. J.
Ophthalmol., 80, 1-23.
30.Mueller-Jensen, K., Machemer, R., and Azarnia, R.
(1975) Am. J. Ophthalmol., 80, 530-537.
31.Skulachev, V. P. (2003) in Topics in Current
Genetics, Vol. 3 (Nystrom, T., and Osiewacz, H. D., eds.)
Springer-Verlag, Berlin-Heidelberg, pp. 191-238.
32.Skulachev, V. P., and Longo, V. D. (2005) Ann.
N. Y. Acad. Sci., 1057, 145-164.
33.Zorov, D. B., Filburn, C. R., Klotz, L. O.,
Zweier, J. L., and Sollott, S. J. (2000) J. Exp. Med.,
192, 1001-1014.
34.Vlachantoni, D., Tulloch, B., Taylor, R. W.,
Turnbull, D. M., Murphy, M. O., and Wright, A. F. (2006) Invest.
Ophthalmol. Vis. Sci., E-5773.
SUPPLEMENTARY INFORMATION
Download (PDF)
SkQ1 Returns Vision to Blind Animals Suffering from Retinal Pathologies (case descriptions)
Below, some examples of successful therapy using drops of 250 nM SkQ1 are presented. In all cases, SkQ1 was used when other kinds of therapy (i.e. instillation of taufor, vitidurol, catachrome, emoxipin, gentamycin or per os treatment with ascorutin, vitamins A and E, riboxin) had already failed.
Case 1. A dog Bagz, dachshund, male, born August 2006, blind due to an inherited retinal dysplasia.
01.02.07. Before SkQ1 treatment. Blinking and pupil reflexes to light are absent, the animal does not orient in a labyrinth, fails to react on a ball moving near the eyes. The major part of tapitum lucidum is dark brown, the rest is blue-green and hyperreflective. Both a- and b-waves on the retinogram are of very much lower magnitude than normal.
09.02.07. 8-day treatment with SkQ1 (one drop of 250 nM SkQ1 solution into each eye daily). Some vision appeared. The dog recognizes the host at a certain distance and reacts to objects appearing near the eyes.
09.03.07. One month of SkQ1 treatment. Light-induced blinking and pupil reflexes as well as reaction to a ball can be shown. The dog orients in a labyrinth but at rather strong illumination only.
24.04.07. 2.5 months of SkQ1 treatment. Appearance of ability to orient in the labyrinth even under dim light. The color of t. lucidim is normalized. The magnitude of waves in electroretinogram is significantly increased.
Case 2. A dog Greta, jackrussel terrier, female, born Nov., 2005. Blind due to an inherited retinal displasia.
12.01.07. Before treatment. Symptoms, state of tapitum lucidum are similar to Case 1 (see above). No light response on electroretinogram (Fig. 11, see color insert for the main text of the article; here and further figures designated without “S” refer to the main article).
17.01.07. Five days of 250 nM SkQ1 instillation. First signs of the seeing behavior appeared.
12.02.07. One month treatment. Like in Case 1.
22.03.07. 42 days treatment. The electroretinogram clearly shows a response to light (Fig. 11).
Case 3. Morgan, a Pyrenean dog, male of two years old was blind due to uveitis pathology.
10.05.06. Before treatment. There are several gray and yellow-brown areas in the retina. Moreover, hyperemia of blood vessels and a hemorrhage of the retina were observed.
23.05.06. 13 days SkQ1 treatment resulted in a partial recovery of vision, reappearance of reaction of the pupil to the light and reduction of all signs of inflammation in the retina and the visual nerve disc.
10.07.06. Treatment continued. The dog recognizes not only large but also small objects.
Case 4. Bonya, a terrier, male, 2, was blind due to a progressing generalized bilateral retinal degeneration.
11.05.06. Before treatment. Depigmentation of t. lucidum (small white-yellow and extensive dark area). Depigmentation of t. nigrum. Peripheral blood vessels are constricted and the retinal nerve disc is white with uneven borders.
23.05.06. 12-day SkQ1 treatment. Vision partially returned. Pupils react to light. The size of dark areas of retinal degeneration is decreased, and blood vessels are dilated. Retinal recovery occurs in a bilateral fashion.
21.06.06. Treatment continued. Further recovery of the retina. Original color of t. lucidum and the optic nerve disc returned. The dog recognizes objects not only in vicinity but also at long distance.
Case 5. A dog Bella, female, toy-terrier, 8. Blind because of progressing retinal degeneration.
05.09.06. Before treatment. The optic nerve disc has uneven borders, and t. nigrum is of a gray-brown color. Initial stage of senile cataract. Behavior and other parameters like in Case 1.
15.09.06. 10-day treatment. The first signs of appearance of vision.
14.10.06. After 40-day treatment. Both vision and the state of retina are normalized.
Case 6. Cat Barsa, female, European short hair race, 15. Blind because of a combined retinal pathologies (retinitis, papillitis, generalized progressing retinal degeneration).
29.05.06. Before treatment. Depigmentation of t. lucidim, the optic nerve disc is violet, exfoliation of retina. No pupil reflex.
09.06.06. 11-day treatment. Appearance of pupil reflex and some visual orientation. The optic nerve disc became pink but exfoliation of retina is still observed.
20.06.06. 20-day treatment. Vision is normalized. Signs of retinal degeneration and exfoliation disappeared.
Case 7. Horse Mashuk, gelding, 20. Blind for 8 months because of a retinal pathology.
15.06.06. Before treatment. Blood vessels in region of the optic nerve are shorter and thinner than normal. Depigmentation of t. lucidum and t. nigrum. Retina is thin. Senile cataract.
10.09.06. 80-day treatment. The color of t. lucidum is normalized. New blood vessels appear in the region of the optic nerve disc. They are of normal length and diameter. The animal recognizes objects even at long distance.
Fig. S1. Dynamics of cataract (a) and retinopathy (b) in OXYS rats. Where indicated, SkQ1 (10, 50, or 250 nmol/kg per day) was added to the food in the period of months 1.5-12.5.
Fig. S2. Effects of SkQ1 on cataract in Wistar and OXYS rats. a, b) Data of two different experiments are shown. 13.5-month-old rats received SkQ1 with food during months 1.5-12.5.
Fig. S3. Electron microscopic study of structure of the retinal pigment epithelium (a, b) and Bruch's membrane (c) of 11 month rats. Effects of 250 nM SkQ1 drops during the last 1.5 months are shown. c) Arrowhead, Bruch's membrane; arrow, hernia formed due to disruption of Bruch's membrane.
Fig. S4. Effect of 20 nM SkQ1 on the macrophagal transformation of RPE cells during a roller cultivation of rat eye posterior sector. A) A part of the tissue at low magnification. Without SkQ1, numerous cells escaping the RPE cell layer (arrows) are seen (a). Their number is much smaller when SkQ1 was added to the cultivation mixture (b). B) Macrophage transformation of the RPE cells. Left, macrophage antigen staining; right, phase contrast. Arrows, macrophage-positive cells; a, b) without SkQ1; c, d) with SkQ1.