Structural–Functional Characteristics of a New Bioregulator Isolated from Bovine Pigmented Epithelium Tissue
V. P. Yamskova1*, V. S. Skripnikova2, A. A. Molyavka2, A. P. Il’ina2, M. S. Krasnov1, D. V. Margasyuk2, A. V. Borisenko2, B. B. Berezin2, E. S. Kuznetsova3, A. K. Buryak3, and I. A. Yamskov2
1Koltzov Institute of Developmental Biology, Russian Academy of Sciences, ul. Vavilova 26, 119334 Moscow, Russia; fax: (499) 135-8012; E-mail: Yamskova-vp@yandex.ru2Nesmeyanov Institute of Organoelemental Compounds, Russian Academy of Sciences, ul. Vavilova 28, 119991 Moscow, Russia; fax: (499) 135-5037; E-mail: Yamskov@mail.ru
3Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, Leninsky pr. 31, 119991 Moscow, Russia; fax: (495) 952-5308; E-mail: tsiv@phyche.ac.ru
* To whom correspondence should be addressed.
Received March 16, 2009; Revision received April 21, 2009
A new bioregulator operating in ultralow doses corresponding to 10–17 mg/ml has been isolated from tissue of pigmented epithelium of bovine eyes. It has been established that the functional basis of this bioregulator is a complex of a low molecular weight regulatory peptide (4372 Da) and a modulator consisting of a mixture of proteins with molecular weights of 14.980-66.283 kDa. It has been shown that the regulatory peptide is responsible for membranotropic activity of the bioregulator, and the modulator proteins are responsible for biological action in ultralow doses. The data demonstrate an interrelation between nanocondition of the bioregulator and its ability to show activity in ultralow doses.
KEY WORDS: bioregulators, regulatory proteins, nanoparticles, ultralow dosesDOI: 10.1134/S0006297909090041
Abbreviations: AFM, atomic-force microscopy; PE, pigmented epithelium; RP, regulatory proteins; TFA, trifluoroacetic acid; ULD, ultralow doses.
The search for previously unstudied endogenous biologically active
substances and identification of their structural features is an
important problem of modern bioorganic chemistry. Some of these
substances are previously unstudied protein bioregulators that have
been found in different animal and plant tissues [1-14]. Their identification has
become possible owing to the development of a new experimental
approach, including the original procedure of isolation (extraction) of
bioregulators from tissues in combination with classical purification
procedures and biotesting method development [1-3]. New models of organotypic cultivation of tissues
in vitro have been developed to study the specific activity of
bioregulators of this group [6-14]. It has been shown that these bioregulators in
ultralow doses (ULD) influence the course and directedness of the most
important biological processes (migration, adhesion, differentiation,
proliferation of cells) and stimulate tissue neogenesis and reparation
[1-3, 6-14]. The bioregulators demonstrate similar
physicochemical properties: they are present as nanoparticles
(50-200 nm) in aqueous solutions; they are composed of low
molecular weight proteins (regulatory proteins, RP); and their activity
is resistant to the action of various physicochemical factors [7-13]. A typical property of
bioregulators of this group is high calcium binding activity [5].
It has been shown that different tissues of vertebrates contain proteins modulating RP activity, termed modulator proteins. One such protein modulating the activity of serum RP was identified as a representative of the multifamily of blood serum albumins [15]. Regulatory protein and modulator interact by the mechanism of lectin–carbohydrate recognition, and the modulator protein binding to the oligomannoside component of RP plays the role of lectin [15].
The study of mammalian eye tissue has shown it to contain bioregulators of this group, the activity of which is characterized by the presence of tissue but absence of species specificity [5, 7]. Bioregulators of the tissues of the back of the eye (retina, pigmented epithelium (PE)) differ from those isolated from other (including eye) tissues, i.e. lens, cornea, and sclera, in the high RP affinity to modulators. Therefore, the bioregulators isolated from retina and PE have been studied previously in fractions containing “RP–modulator” complexes [5, 6]. These studies show that the bioregulator isolated from PE has an effect on stabilization of differentiation of PE cells of the eye of adult tritons and maintains the viability of bipolar and Muller cells in vitro [6]. However, the regulator isolated from neutral retina, i.e. tissue homogenous to and functionally associated with PE, had no effect on the state of PE cells but contributed to the maintenance of viability of Muller glia cells in vitro [6].
In the present work, conditions for separation of RP–modulator complex have been found and the separate components of the complex have been obtained, the biological activities of RP fractions, modulator, and RP–modulator complex have been studied, and compositions of the components of the complex have been assessed using mass spectrometry. The bioregulator isolated from eye PE has been studied as well.
MATERIALS AND METHODS
Bioregulator isolation and purification. Pigmented epithelium was preparatively separated from other tissues of freshly denucleated eyes of young bull calves (Tagansky Meat Processing Industrial Complex, Russia) and put for 3 h at 4°C into an extracting solution containing: NaCl, 0.15 M; CaCl2, 1 mM; Hepes, 1 mM. After centrifugation of the tissue extract at 3000g for 30 min, the supernatant was collected and salted out by the use of dry ammonium sulfate (780 g/liter) in the presence of 10–2 M EDTA. The resulting protein suspension was centrifuged at 105,000g for 45 min, and the following three fractions were collected: (1) supernatant; (2) floating fraction; and (3) precipitate. Then these fractions were dialyzed against water to complete elimination of ammonium ions, and the membranotropic activity was determined by adhesiometry [3].
Reversed-phase high performance liquid chromatography of PE supernatant fraction was carried out using an Agilent 1100 Series high-pressure liquid chromatographer (USA) using BioKhimMak C8 column (4.6 × 150 mm, 200 Å). Tested sample, 100 µl, was applied to the column. A 0.1% trifluoroacetic acid (TFA)–acetonitrile mixture was used as an eluent; the mixture was fed to the column in the acetonitrile concentration gradient of 5 to 80% at a rate of 0.5 ml/min at 25°C. Protein fractions were detected at 210 and 280 nm.
The hydrophilic PE fraction with retention time 3.1 min was rechromatographed on a Luna C18 column (2 × 150 mm, 100 Å) (Phenomenex, USA) pre-equilibrated in the starting buffer (0.1% TFA). Chromatography was carried out at a rate of 0.3 ml/min in a gradient of acetonitrile concentration 0→30% during 70 min. The yield of fractions was determined using spectrophotometry, by optical absorption at 210 and 280 nm. The resulting fraction (retention time 59.8 min) was harvested and lyophilized, followed by mass-spectrometric analysis.
Reversed-phase HPLC of the components obtained after PAGE of the hydrophobic fraction with retention time 14.23 min was carried out on a column of the Jupiter C5 series (2 × 150 mm, 300 Å) (Phenomenex) pre-equilibrated with the starting buffer (0.1% TFA). Chromatography was carried out at a rate of 0.3 ml/min using the linear acetonitrile gradient: 0→70% during 70 min. The yield of fractions was determined using spectrophotometry by the optical absorption at 210 and 280 nm. The resulting fractions were harvested and lyophilized, followed by mass-spectrometric analysis.
SDS-PAGE was carried out according to Laemmli [16] in vertical polyacrylamide gel plates, 0.75 mm thick, with acrylamide concentrations of 15 and 5% for separating and concentrating gel, respectively. Electrophoresis was carried out for 1 h under DC, 20-30 mA. After completion of the electrophoresis, the gel was fixed and stained with Coomassie R250. Fragments corresponding to proteins with molecular masses of 67-70, 60-66, and 30-35 kDa were cut out. Marker proteins of 14.4-94 kDa were used (Helicon, Russia).
Affinity chromatography was used to study two fractions obtained after the reversed-phase chromatography of the supernatant isolated from PE tissue extract. This examination followed the procedure developed previously during the study of RP and modulator protein isolated from cattle blood serum [15]. The hydrophilic fraction (4 ml) was applied by recirculation to a column with ConA-Sepharose 4B at 4°C for 100 h at the rate of 1-2 ml/min. The sorbent was washed with 0.15 M NaCl for 36 h, and then RP was eluted for 100 h by recirculation feed of a solution containing α-methyl-D-mannoside, 1 M; CaCl2, 10 mM; MnCl2, 0.1 mM; and sodium azide, 0.1 mM. Thus resulting fraction was harvested, dialyzed against solution containing 10 mM CaCl2, and used for determination of membranotropic activity.
The hydrophobic fraction (10 ml) was applied by recirculation to a column with mannose-Sepharose 4B at 4°C during 100 h at a rate of 1-2 ml/min. Then the sorbent was washed with 0.15 M NaCl for 36 h, and the modulator protein was eluted by recirculation feed during 100 h of a solution containing α-methyl-D-mannoside, 1 M; CaCl2, 10 mM; MnCl2, 0.1 mM; and sodium azide, 0.1 mM. The resulting protein fraction was harvested and dialyzed against the solution containing 10 mM CaCl2; the membranotropic activity was determined.
MALDI-TOF spectrometry. Molecular masses of proteins were analyzed by the MALDI-TOF method on a UltraFlex 2 time-of-flight mass spectrometer (Bruker Daltonic, Germany). The time-of-flight mass spectra were fixed in the linear mode and in the reflector mode. The samples for mass-spectrometric analysis were dried and the residue was dissolved in 70% acetonitrile containing 0.1% TFA to obtain no less than 10 pmol/µl solution. The matrices used in the work were sinapic acid and 2-cyano-4-hydroxycinnamic acid.
Size of particles in PE bioregulator solutions was determined by the methods of dynamic laser light scattering [17] and atomic-force microscopy (AFM) [18]. Specific activity of PE bioregulator and its components was studied with a model of organotypic cultivation of PE as a component of the back of the eye of the triton Pleurodeles waltl [6]. Mature adult individuals of both sexes were taken from the aquarium of the Koltzov Institute of Developmental Biology, Russian Academy of Sciences. No less than 10 animals (20 eyes) were used in each cultivation experiment. Tritons were narcotized in 0.1% MS-222 solution.
The medium for eye tissue isolation and cultivation contained: 350 ml of medium 199, 150 ml of bidistilled water, 0.15 ml of 1 M Hepes buffer, and 1 ml of 4% gentamycin sulfate solution. Before placing into vials, the cultivation medium was exposed to cold sterilization through GV membrane filter with the pore diameter of 0.22 µm (Millipore, USA).
Triton eye tissues were isolated using a binocular microscope in the following sequence. First, an eye was released from cutaneous covering and fat and then cut along the circumference, more proximally than the limbus. The growth zone of the retina together with iris, cornea, and lens was removed. The back of the eye including retina, PE, vascular membrane, and sclera was used for subsequent cultivation. The obtained back of the eye was placed into penicillin vials filled with serum-free medium on A/E cellulose filters (Gelman, USA) washed in 70% ethanol and rinsed in the culture medium. The fraction of the bioregulator or its components in tested concentrations was added into experimental vials. In the control vials, the corresponding amount of saline solution was added to the culture medium.
All vials were closed with sterile caps and then with Parafilm M, covered with aluminum foil, and put into a thermostat. Cultivation was carried out under stationary conditions in the dark at 20-22°C for 3 days. The culture medium was not replaced during that period.
RESULTS AND DISCUSSION
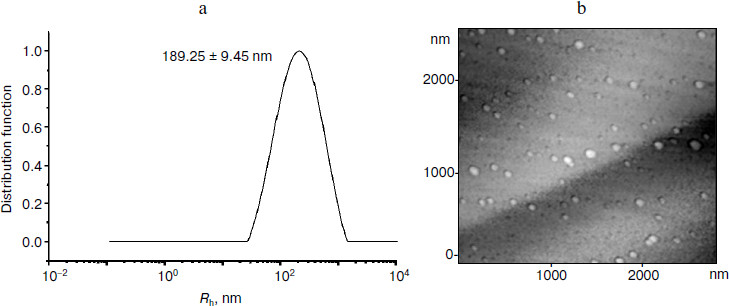
As a result of salting-out of PE tissue extract (37.5 mg protein) by ammonium sulfate in the presence of EDTA, the following three factions were obtained: the supernatant, the precipitate, and the fraction floating after centrifugation. The biotesting showed that the precipitate fraction was inactive, so it was not studied in further experiments. Membranotropic activity was exhibited by two fractions: the supernatant and the floating fraction in the concentrations corresponding to 10–11 and 10–14-10–15 mg/ml. Protein amount was 0.5 mg in the supernatant and 20 mg in the floating fraction. Dynamic laser light scattering and AFM showed that the water solutions of the supernatant and floating fraction contained particles of 100-200 nm (Fig. 1).
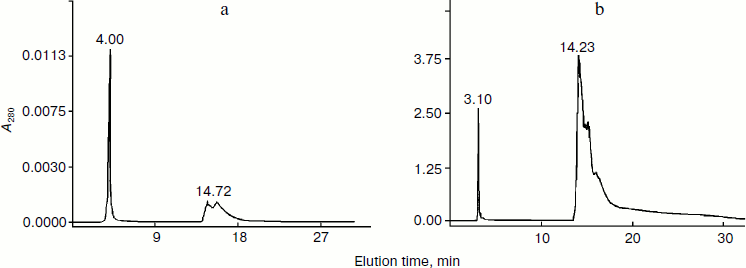
The same situation was observed after separation of both the supernatant and the floating fraction by the method of reversed-phase HPLC: two resulting fractions were identified as hydrophilic (retention time 3-5 min, protein amount corresponding to 0.035 mg for the supernatant and to 0.046 mg for the floating fraction) and hydrophobic (retention time 14-17 min, protein amount corresponding to 0.02 mg for the supernatant and to 0.2 mg for the floating fraction) (Fig. 2). The method of biotesting showed that the membranotropic activity was exhibited by the former fraction only, but in concentrations higher than ULD and corresponding to the ligand–receptor mechanism (10–5-10–6 mg/ml) [19] (Fig. 3). The latter fraction showed no membranotropic activity. The findings demonstrate that the hydrophilic fraction displays membranotropic activity in a higher concentration as compared with the initial fractions – floating and supernatant. It should be noted that nano-sized protein aggregates were not found in the aqueous solutions of these fractions.Fig. 1. Study of particle size in the supernatant fraction isolated from pigment epithelium by dynamic laser light scattering (a) and atomic force microscopy (b). a) Distribution of particles (scattering angle 90º) present in the aqueous solution of the supernatant fraction isolated from PE. Rh, hydrodynamic radius. b) Particles of the supernatant fraction isolated from PE on the mica chip surface (contact mode scanning). Axes, linear particle sizes, nm.
Fig. 2. Reversed-phase HPLC of the supernatant (a) and floating at 105,000g (b) fractions isolated from PE.
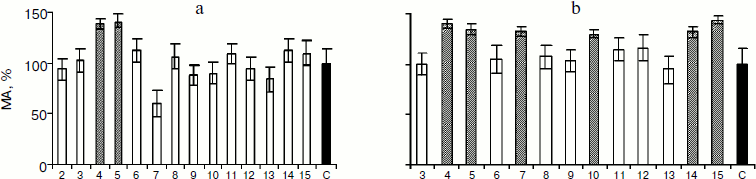
Previously, the study of the bioregulator isolated from cattle blood serum has shown the presence in the latter of a protein modulating the activity of serum RP [15]. Based on those results, it has been supposed that the bioregulator isolated from PE also contains a modulator influencing the RP activity, which is present in the hydrophobic HPLC fraction. The method of biotesting showed that the RP–modulator complex restored by way of combining both HPLC fractions in the presence of Ca2+ exhibited the membranotropic activity in ULD, which was of polymodal character (Fig. 3). The results show that the hydrophobic fraction influences the membranotropic activity of RP and can be considered as its modulator [15]. An important result is the finding of protein aggregates of 100-300 nm in the aqueous solution of the RP–modulator complex isolated from PE.Fig. 3. Membranotropic activity (MA) of regulatory peptide (a) and regulatory peptide–modulator complex (b) isolated from PE in vitro. X-Axis, degree of tenfold dilution of the given protein fraction (initial concentration 0.046 (a) and 0.026 mg/ml (b)). C, control; columns – the effect of fraction in 10x dilution, where “x” is index of the number of tenfold dilution of tested preparation. Shading is to indicate the columns that correspond to the dilutions of fractions where membranotropic activity was found.
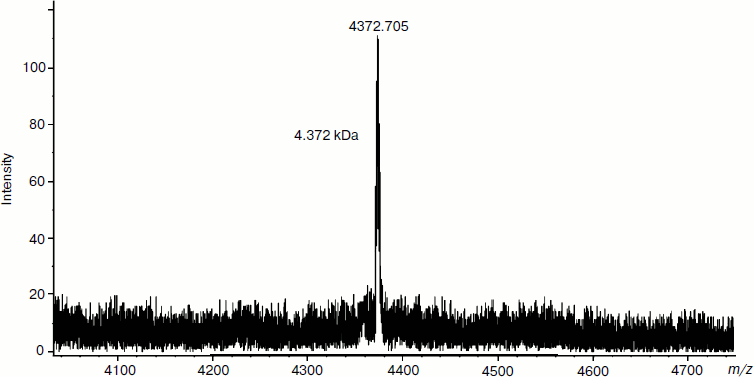
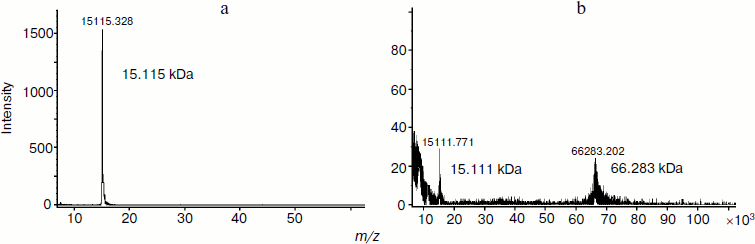
It was interesting to study the components of the hydrophilic HPLC fraction (retention time 3-5 min) and hydrophobic HPLC fraction (retention time 14-17 min). This was done by MALDI-TOF mass spectrometry. With this purpose, the HPLC fraction with retention time 3-5 min isolated from PE tissue extract was rechromatographed on the C18 column. MALDI-TOF mass spectrometry was used to study the HPLC fraction with retention time 59.8 min (Fig. 4). According to the results, the identified signal corresponds to a peptide with molecular mass of 4372.705 Da. It should be noted that no other signals equivalent in intensity were revealed. These data indicate, first, the high degree of purification of this fraction obtained as a result of repeated reversed-phase HPLC and, second, the presence of a peptide with the mentioned mass.
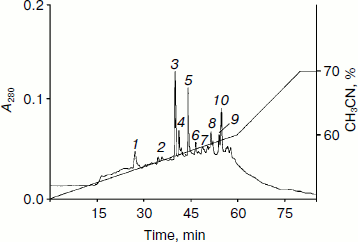
For identification of proteins composing the HPLC fraction with retention time 14-17 min, this fraction was separated by PAGE. The molecular mass values of three isolated fractions corresponded to 67-70, 60-66, and 30-35 kDa. These fractions were separated by reversed-phase HPLC on the C5 column (Fig. 5). The analysis of chromatographic elution profiles of the studied fractions showed the complete identity of these three fractions. The most quantitatively represented components (marked by numerals in Fig. 5) were collected for the study of homogeneity and molecular mass by MALDI-TOF mass spectrometry. The main components of peaks 4-7 are proteins with the molecular masses of 14980.432, 15108.818, 15115.328, and 15101.022 Da, respectively. The mass spectrum of peak 8 is characterized by the presence of two signals corresponding to the molecular masses of 15141.220 and 29269.781 Da. The mass spectra of peaks 9 and 10 are identical; their major components are two proteins with molecular masses of 15111.771 and 66283.202 Da. Figure 6 (a and b) exemplifies mass-spectra of peaks 6 and 10, respectively.Fig. 4. Regulatory peptide mass spectrum obtained by MALDI-TOF mass spectrometry on an Ultraflex 2 (Bruker, Germany).
Fig. 5. Reversed-phase HPLC in water–acetonitrile gradient (0-70%) of the polyacrylamide gel-eluted fraction isolated from PE.
The results show that the major components of all identified peaks are proteins with molecular mass values varying around 15 kDa. These are probably isoforms of a single protein or a few proteins having different chromatographic mobility under the conditions of reversed-phase HPLC. It should be noted that the identified high molecular weight protein (66283.202 Da) corresponds to BSA in molecular mass.Fig. 6. Mass spectra of the proteins isolated from PE (peaks 6 (a) and 10 (b) obtained by MALDI-TOF mass spectrometry on Ultraflex 2).
Thus, the MALDI-TOF mass spectrometric data has shown that the PE bioregulator includes a regulatory peptide (4372.705 Da) possessing membranotropic activity. The modulator fraction contains proteins with molecular mass values within 14980-66283 Da. The yield of target proteins used after rechromatography for the analysis by MALDI-TOF mass spectrometry was 0.8-2.0 µg.
These data are in agreement with the results of the study of cattle blood serum bioregulator showing that its biologically active component is a small peptide (with molecular ion value of 1338.7 Da) whose activity in blood serum is reversibly inactivated by the modulator, which is a previously unstudied protein – representative of the multifamily of blood serum albumins [3, 15]. The affinity chromatography used in this research showed that the interaction between serum regulatory peptide and its modulator proceeded by the mechanism of carbohydrate–protein interaction. In the present work, the molecular mechanism underlying the interaction of regulatory peptide and the modulator isolated from PE has also been studied by affinity chromatography [15]. The hydrophilic fraction (retention time 3-5 min) was studied in a column with Sepharose 4B containing immobilized concanavalin A (ConA-Sepharose 4B); the hydrophobic fraction (retention time 14-17 min) was studied in a column with Sepharose 4B containing immobilized mannose (man-Sepharose 4B).
The fractions, collected after the affinity chromatography, were studied by the method of biotesting. It was shown that membranotropic activity was exhibited by the fraction obtained after chromatography on a sorbent, which was ConA immobilized on Sepharose 4B, and only in the concentrations corresponding to 10–5-10–6 mg/ml. After the interaction of this fraction and the fraction obtained after chromatography on mannose immobilized on Sepharose 4B, the membranotropic activity of the formed complex was exhibited in ULD and had polymodal dose dependence.
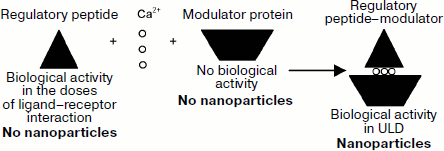
The findings demonstrate that the interaction between regulatory peptide of the eye PE and the modulator develops by the carbohydrate–protein mechanism, according to which the modulator plays the role of lectin that binds the carbohydrate component of regulatory peptide. It should be noted that regulatory peptides have been found in all bioregulators of this group identified in different mammalian tissues [7-9, 11-13] and the existence of their modulators in tissues has been shown as well [1, 2, 4, 8]. These data suggest that, first, there is a single mechanism of interaction of regulatory peptides of this group with their modulators (Scheme), and, second, the character of action of modulators on regulatory peptide activity is determined by the functional peculiarities of respective tissue. For example, the serum bioregulator is present in blood in the inactive state due to the interaction of regulatory peptide and the modulator protein [3, 15], and the formation of regulatory peptide complex with modulators determines the tissue-specific character of activity for the bioregulators isolated from liver, lung, cornea, and eye lens [7-9, 11-13]. In case of tissues of the back of the eye, including the bioregulator isolated from PE, it was unclear how the formation of regulatory peptide–modulator complex influenced the specific activity of this bioregulator. Hence, we have studied the specific activity of regulatory peptide, modulator, and restored regulatory peptide–modulator complex isolated from PE using the model of stationary cultivation of tissues of the back of the eye of adult tritons Pleurodeles waltl [7]. The bioregulator isolated from PE has been chosen as an object of research because the state of PE monitored by light microscopy makes it possible to assess simply but reliably the state of adhesion, differentiation status, proliferative activity, and viability of retina pigment cells.
The following fractions were examined: hydrophilic HPLC fraction in a dose corresponding to the concentration of 10–7 mg protein/ml; hydrophobic HPLC fraction in a dose corresponding to 10–7 mg protein/ml; regulatory peptide–modulator complex in a dose corresponding to 10–7 mg protein/ml; regulatory peptide–modulator complex in a dose corresponding to 10–17 mg protein/ml.
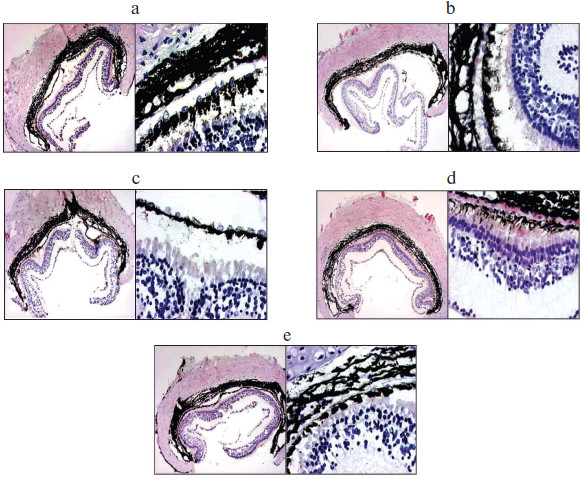
Cultivation was carried out for 3 days. During that time, gradual degradation of all eye tissues took place in the control (Fig. 7a; see color insert). The retina contained a considerable number of apoptotic cells, the adhesion between the retina, PE, and choroid was disturbed, and the state, i.e. differentiation status, of PE cells was changed as assessed by the shift of pigment to the apical side of monolayer cells, disturbance of adhesion between them, and cell death. The formation of noticeable cavities was noted in sclera as a result of degradation of collagen fibers.
The regulatory peptide-containing hydrophilic HPLC fraction had a certain protective effect on the state of PE. The contact interactions between PE and choroid were maintained, though detachment of the retina took place in any case. In the monolayer, PE cells looked more viable than in the control: the nuclei were rounded, with evenly distributed chromatin, and the shift of pigment in the cell cytoplasm was not so marked. The sclera retained spatial arrangement of collagen fibers and had no cavities (Fig. 7b).Fig. 7. Microphotographs of tissues of the back of the eye of triton Pl. waltl after 3-day stationary cultivation. Left, magnification ×40; right, magnification ×200. a) Without the bioregulator isolated from pigment epithelium (control); b) with the regulatory peptide isolated from pigment epithelium, at a concentration of 10–7 mg/ml (experiment); c) with the modulator isolated from pigment epithelium, at a concentration of 10–7 mg/ml (experiment); d) with the regulatory peptide–modulator complex isolated from pigment epithelium, at a concentration of 10–17 mg/ml (experiment); e) with the regulatory peptide–modulator complex isolated from pigment epithelium, at a concentration of 10–7 mg/ml (experiment).
The state of tissues of the back of the eye after cultivation with addition of hydrophobic modulator-containing HPLC fraction corresponded to the state of tissues of the back of the eye in the control (Fig. 7c).
On cultivation of tissues of the back of the eye with the addition of regulatory peptide–modulator complex in a dose corresponding to 10–7 mg/ml (ligand–receptor interaction), the effect on tissue state in the experiment was less marked (Fig. 7e). In contrast to the control, there were adhesive interactions between retina, PE, and choroid in an extensive area of the back of the eye with insignificant disturbances in some regions. Most retina cells were in the apoptotic state as in the control. It should be noted that in the monolayer of PE cells, adhesive interactions were maintained and pigment granules were evenly distributed in the cytoplasm of cells, indicating stable differentiation of these cells under the above conditions as compared with the control. The state of scleral coat did not differ from the control.
Quite another situation was observed on addition of the regulatory peptide–modulator complex to the medium in ULD (10–17 mg protein/ml) (Fig. 7d). This experiment revealed the maximum integrity of tissues of the back of the eye. Close adhesive interactions were registered between photoreceptor processes, PE monolayer, and choroid. In contrast to other experiments, the number of apoptotic cells in the retina was insignificant. The status of PE cell differentiation stabilized as shown by the even distribution of pigment in these cells and maintenance of intact monolayer structure. The state of sclera tissue was close to that of intact tissue: the compact structure of collagen fibers was preserved.
Thus, the regulatory peptide–modulator complex isolated from PE has the most marked protector effect on the tissues of the back of the eye in ULD (10–17 mg protein/ml).
The study of biological activity of the fractions containing PE regulatory peptide, modulator, and regulatory peptide–modulator complex shows that the bioregulator has a complicated structure. It comprises a regulatory peptide responsible for membranotropic effect of the bioregulator and a modulator influencing the regulatory peptide activity. The regulatory peptide–modulator complex formed by calcium ions is a functional basis of the PE bioregulator. The significance of nanocondition of the bioregulator isolated from pigment epithelium has been demonstrated by exhibition of its activity, both membranotropic and specific, in ultralow doses.
REFERENCES
1.Yamskova, V. P., Modyanova, E. A., Levental, V. I.,
Lankovskaya, T. P., Bocharova, O. K., and Malenkov, A. G. (1977)
Biofizika, 22, 168-174.
2.Yamskova, V. P., Modyanova, E. A., Reznikova, M.
M., and Malenkov, A. G. (1977) Mol. Biol. (Moscow), 11,
1147-1154.
3.Yamskova, V. P., and Reznikova, M. M. (1991) Zh.
Obshch. Biol., 52, 181-191.
4.Yamskov, I. A., Yamskova, V. P., Danilenko, A. N.,
Klemenkova, Z. S., Antipov, B. G., Chernikov, F. R., Gusynina, M. M.,
and Rybakova, E. Yu. (1999) Zh. Ros. Khim. Obshch. im. D. I.
Mendeleeva, 43, 34-39.
5.Krasnov, M. S., Grigoryan, E. N., Yamskova, V. P.,
Boguslavsky, D. V., and Yamskov, I. A. (2003) Radiats. Biol.
Radioekol., 3, 265-268.
6.Krasnov, M. S., Grigoryan, E. N., and Yamskova, V.
P. (2003) Vestnik RAN. Ser. Biol., 1, 22-36.
7.Krasnov, M. S., Gurmizov, E. P., Yamskova, V. P.,
and Yamskov, I. A. (2007) in Biochemical Physics Frontal Research
(Varfolomeev, S. D., Burlakova, E. B., Popov, A. A., and Zaikov, G.
E., eds.) Nova Science Publishers Inc., Hauppauge-N. Y., pp. 21-34.
8.Borisenko, A. V., Yamskova, V. P., Krasnov, M. S.,
Blagodatskikh, I. V., Vecherkin, V. V., and Yamskov, I. A. (2007) in
Biochemical Physics Frontal Research
(Varfolomeev, S. D., Burlakova, E. B., Popov, A. A., and Zaikov, G.
E., eds.) Nova Science Publishers Inc., Hauppauge-N. Y., pp.
35-46.
9.Margasyuk, D. V., Krasnov, M. S., Blagodatskikh, I.
V., Grigoryan, E. N., Yamskova, V. P., and Yamskov, I. A. (2007) in Biochemical Physics Frontal Research
(Varfolomeev, S. D., Burlakova, E. B., Popov, A. A., and Zaikov, G.
E., eds.) Nova Science Publishers Inc., Hauppauge-N. Y., pp. 47-59.
10.Yamskova, V. P., Krasnov, M. S., Rybakova, E.
Yu., Vecherkin, V. V., Borisenko, A. V., and Yamskov, I. A. (2007) in
Biochemical Physics Frontal Research (Varfolomeev, S. D.,
Burlakova, E. B., Popov, A. A., and Zaikov, G. E., eds.) Nova Science
Publishers Inc., Hauppauge-N. Y., pp. 71-78.
11.Nazarova, P. A., Yamskova, V. P., Krasnov, M. S.,
Filatova, A. G., and Yamskov, I. A. (2007) in New Trends in
Biochemical Physics Research (Varfolomeev, S. D., Burlakova, E. B.,
Popov, A. A., and Zaikov, G. E., eds.) Nova Science Publishers Inc.,
Hauppauge-N. Y., pp. 73-82.
12.Yamskova, V. P., Rybakova, E. Yu., Vecherkin, V.
V., Berezin, B. B., Filatova, A. G., Blagodatskikh, I. V., and Yamskov,
I. A. (2007) in Biochemical Physics Frontal Research
(Varfolomeev, S. D., Burlakova, E. B., Popov, A. A., and Zaikov, G.
E., eds.) Nova Science Publishers Inc., Hauppauge-N. Y., pp. 61-70.
13.Skripnikova, V. S., Krasnov, M. S., Berezin, B.
B., Babushkina, T. A., Borisenko, A. V., Izmaylov, B. A., Yamskova, V.
P., and Yamskov, I. A. (2007) Vestnik Akad. Nauk, 417,
697-699.
14.Krasnov, M. S., Gurmizov, E. P., Yamskova, V. P.,
Gundorova, R. A., and Yamskov, I. A. (2005) Byul. Oftalmol.,
121, 37-39.
15.Yamskova, V. P., Rybakova, E. Yu., Vinogradov, A.
A., Vecherkin, V. V., and Yamskov, I. A. (2004) Prikl. Biokhim.
Mikrobiol., 40, 407-413.
16.Laemmli, U. K. (1970) Nature, 227,
680-685.
17.Stepanek, P. (1993) Dynamic Light Scattering.
The Method and Some Applications (Brown, W., ed.) Clarendron Press,
Oxford, p. 171.
18.Filonov, A. S., Gavrilko, D. Yu., and Yaminsky,
I. V. (2005) Software for 3D Image Processing “FemtoScan
Online”, Center for Advanced Technologies, Moscow, 89, (http://www.nanoscopy.net).
19.Burlakova, E. B., Konradov, A. A., and Maltseva,
E. L. (2004) Biofizika, 49, 551-564.