Effect of Ficoll 70 on Thermal Stability and Structure of Creatine Kinase
Yejing Wang1, Huawei He2, and Sen Li3*
1School of Life Sciences, Lanzhou University, Lanzhou 730000, Gansu, P. R. China2Department of Biological Sciences and Biotechnology, Tsinghua University, Beijing 100084, P. R. China
3Department of Biochemistry and Molecular Biology, Beijing Normal University, Beijing Key Laboratory, Beijing 100875, P. R. China; fax: 86-10-5880-7720; E-mail: lisen@bnu.edu.cn
* To whom correspondence should be addressed.
Received January 27, 2010; Revision received February 24, 2010
The effect of Ficoll 70 on the thermal stability and structure of creatine kinase (CK) was studied using far-UV CD spectra and intrinsic fluorescence spectra. The thermal transition curves monitored by CD spectra were fitted to a two-state model using a modified form of the van’t Hoff equation to obtain the transition temperature (Tm) and enthalpy change (ΔHu) of thermally induced denaturation of CK in the absence and presence of Ficoll 70. An increase in Tm with constant ΔHu was observed with increasing Ficoll 70 concentration, suggesting that Ficoll 70 enhances the thermal stability of CK. Fluorescence spectral measurements confirmed this protective effect of Ficoll 70 on CK structure. In addition, we observed a crowding-induced compaction effect on the structure of both native state and thermally denatured state of CK in the presence of Ficoll 70, which is more obvious on the structure of the denatured ensemble compared to that of the native ensemble. Our observations qualitatively accord with the predictions of previously proposed crowding theory for the effect of intermolecular excluded volume on protein stability and structure. These findings imply that the effects of macromolecular crowding are essential to our understanding of protein folding and unfolding occurring in vivo.
KEY WORDS: macromolecular crowding, thermal stability, Ficoll 70, creatine kinaseDOI: 10.1134/S0006297910050160
Abbreviations: CK, creatine kinase.
In the past four decades since the pioneering studies of Anfinsen [l],
protein folding and unfolding in vitro have been extensively
studied with low concentrations of proteins in simple defined buffers.
This environment differs substantially from that encountered within
cells where proteins have evolved to function. A distinctive property
of living systems is that biochemical processes proceed in a medium
containing high concentrations of macromolecules (50-400 mg/ml
proteins, carbohydrates, nucleic acids, lipids, etc.) [2-5]. The concentration of a
single macrosolute species is not so high, but overall the
macromolecules present in the cell occupy a significant part of the
total volume of the medium (about 40%). Therefore, the accessible
volume in the cell is reduced. In the literature, such conditions in
the living cell have been termed “macromolecular crowding”
[6]. Experimental and theoretical work have
demonstrated large effects of macromolecular crowding on the
thermodynamics and kinetics of many biological processes, including
protein and nucleic acid diffusion, molecular recognition, and protein
folding and assembly [7-11].
Statistical thermodynamic models have shown that the excluded volume effects arising from nonspecific steric repulsion in a crowded macromolecule medium can play a significant role in the function, stability, and interaction of the individual macromolecule [12]. By excluding a part of the available volume, macromolecules decrease the configurational entropy, resulting in an increase in the chemical potential and free energy of all molecules present in that solution. Thus, macromolecular crowding perturbs the equilibrium of a reaction by destabilizing the reactants or product so as to exclude the least volume to other molecules in the milieu [13]. Given the high concentrations of macromolecules inside cells, folding and unfolding stability of a protein in the cytosol might well be different from what is measured in typical dilute-solution experiments [14]. An equilibrium statistical–thermodynamic model developed primarily by Minton [15] predicts that macromolecular crowding should increase protein thermal stability (Tm) by a magnitude of 5-20°C under physiological solute conditions. Some experimental facts have been found to support this theory: the thermal midpoint of G-actin was found to increase by 5°C in the presence of 100 mg/ml poly(ethylene glycol) 6000 [16]; the presence of 300 mg/ml dextran raised Tm by nearly 3°C for lysozyme at pH 2 [17]; and Ficoll 70 was found to has a concentration-dependent effect on the thermal stability of apoflavodoxin (ΔTm of 20°C at 400 mg/ml, pH 7) [18]. However, more experimental evidence is desirable to prove further the correctness of this theory. Therefore, addressing the effect of crowding on protein stability experimentally is of great current interest.
In this study, we examined the effect of Ficoll 70 on the structure and thermal stability of creatine kinase (ATP:creatine N-phosphotransferase, EC 2.7.3.2, CK). Creatine kinase, a member of the guanidino kinase family, catalyzes the reversible transfer of a phosphoryl group from ATP-Mg2+ to creatine, producing phosphocreatine and ADP-Mg2+ [19]. CK plays an important role in the rapid regeneration of ATP in cells where energy demands are high, and it is used as an important diagnostic indicator for diseases of the nervous system, the heart muscle, and other systems. CK has also been used as a model protein in catalytic, structural, and folding studies for more than five decades. The unfolding and refolding of CK has been studied extensively in the presence of urea and guanidine-HCl and versus temperature [20-23]. Usually macromolecular crowding can be mimicked experimentally by adding high concentrations of inert synthetic or natural macromolecules, termed crowding agents, to the systems in vitro. In this study, Ficoll 70 was used as a model crowding agent because it is an inert, polar polysaccharide that behaves like a semirigid sphere. Thus it is an attractive mimic of globular macromolecules that can be present in the biological setting where protein folding normally takes place. Also, it showed no detectible binding to CK (data not shown). The thermal stability and structural changes of CK were investigated over extended concentrations of Ficoll 70, which could simulate the effect of intracellular crowding on protein stability and structure. Our experimental results are in excellent agreement with the theoretical prediction and demonstrate that macromolecular crowding increases protein thermal stability via the excluded volume effect.
MATERIALS AND METHODS
The preparation of rabbit muscle CK was as described previously [24]. The purified CK was homogeneous on SDS-PAGE. The enzyme concentration was determined by the absorbance at 280 nm with A1 cm1% = 8.8 [24, 25]. Ficoll 70 (average molecular mass 70,000) was purchased from Sigma (USA). All other chemicals were local products of analytical grade. All protein samples were prepared by dissolving the proteins in 10 mM Tris-HCl buffer, pH 8.0. The final concentrations of CK were 0.12 mg/ml (1.4 µM) for most experiments unless mentioned separately.
CD spectral measurements. The CD spectra were measured using a Jasco J-715 spectropolarimeter (Hitachi, Japan) between 200 and 250 nm in quartz cells with 1 mm pathlengths, a resolution of 0.5 nm, and a bandwidth of 2 nm. Equilibrium thermally induced transition curves of CK in the presence of various Ficoll concentrations were monitored at 222 nm. Samples were heated at intervals of 2 K from 298 K (25°С) to 353 K (80°С) and equilibrated for 2 min at each given temperature before the spectral data were recorded. During the CD measurements, the temperature surrounding the cell was maintained within ±0.1 K by circulating water from an RTE-111 temperature-controlled bath (Neslab), and the cells were sealed with Teflon stoppers. All the protein spectra were corrected for the contributions of the appropriate backgrounds (buffer for the protein solution or Ficoll 70 at the corresponding concentration for the Ficoll/protein samples). Samples with a particular concentration of Ficoll 70 were prepared at least in duplicate.
Fluorescence spectral measurements. Thermally induced unfolding of CK in the presence of various concentrations of Ficoll 70 was also monitored with fluorescence spectra. Sample preparation and heat denaturation experiments were performed the same as for the CD measurements. All fluorescence spectra were recorded on a Hitachi F2500 spectrofluorometer (Japan) using a 1-cm pathlength cuvette with an excitation wavelength of 295 nm, which specifically excites the tryptophan residues of CK.
RESULTS
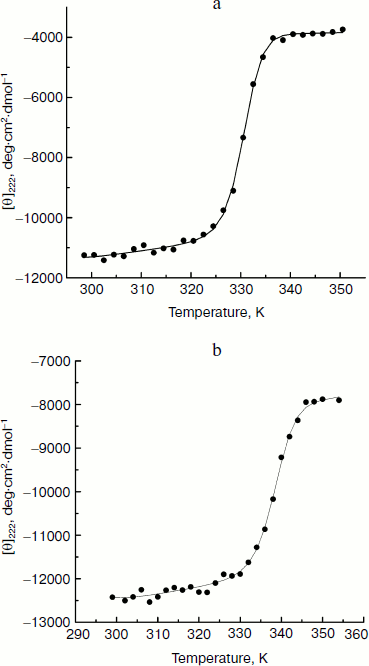
Measurement and analysis of CD spectra for effect of Ficoll 70 on CK. Figure 1 (a and b) shows the thermally induced denaturation curves of CK in the absence and presence of 240 mg/ml Ficoll 70, monitored by determining 222 nm ellipticities in CD spectra, which indicates the secondary structure of CK, as a function of temperature. Analysis of the CD spectra using singular value decomposition [26] shows that each spectrum measured at a particular temperature and Ficoll concentration could be well described by a weighted linear combination of native and denatured state components. Therefore, the dependence of the ellipticity on temperature can be analyzed in the context of a two-state model, where the observed ellipticity at any given temperature is taken as a linear function of the ellipticities of the fully denatured and native state.
The thermally induced denaturation curves in the absence and presence of different concentrations of Ficoll 70 were fitted to a modified form of the van’t Hoff equation that simultaneously fits the baselines of native and denatured state and the transition region of the thermally induced denaturation curves [27]:Fig. 1. Thermally induced transition curves of CK in the absence (a) and presence (b) of 240 mg/ml Ficoll 70. The [θ] of far-UV CD was monitored at 222 nm. The lines show the best fits to a modified van’t Hoff equation as described in the text.
[θ](T) = [anT + bn + (adT + bd)K)]/[1 + K], (1)
where
K = exp(–ΔGu/RT). (2)
Here [θ](T) is the observed CD signal at a given temperature T, an, bn, and ad, bd are the slopes and intercepts of the baselines of native and denatured-state, respectively, K is the equilibrium constant, R is the gas constant, and T is the absolute temperature. ΔGu is the Gibbs free energy change between native and denatured states of CK at a given temperature T, which is assumed to follow the Gibbs–Helmholtz equation [28]:
ΔGu = ΔHu(1 − T/Tm) − ΔCu[(Tm − T) + Tln(T/Tm)]. (3)
In Eq. (3), Tm is the transition temperature at which ΔGu = 0, ΔHu is the enthalpy change of thermally induced unfolding at Tm, and ΔCu is the heat capacity change of thermally induced unfolding.
Data were fitted using the Origin 7.0 program (Origin Lab Corp., USA) using nonlinear least-squares regression analysis. In this nonlinear analysis, seven parameters were used to fit each data set: an, bn, ad, bd, ΔHu, Tm, and ΔCu.
The continuous lines in Fig. 1 (a and b) represent the best fit of the data set to the two-state model based on Eqs. (1)-(3). The calculated best-fit curves and the experimental results are in good agreement within the experimental error. The goodness of fit shown in Fig. 1 (a and b) is comparable to those obtained for all concentrations of Ficoll 70 studied (data not shown). Thus, the two-state model is sufficient to describe the thermally induced unfolding of CK in the presence of Ficoll 70 up to 260 mg/ml.
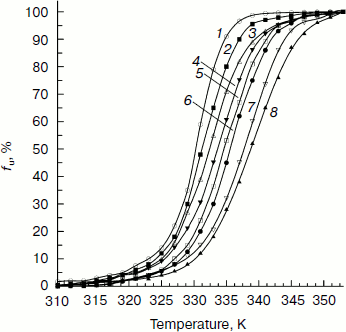
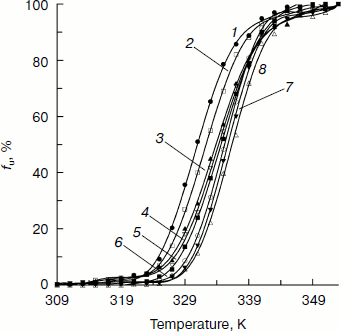
The fraction of unfolded CK, fu, versus temperature calculated according to Eq. (4) is plotted in Fig. 2:
fu = (anT + bn − [θ](T))/(anT + bn − adT − bd). (4)
The transition curves in Fig. 2 clearly indicate that the secondary structure of CK unfolds in a cooperative process during thermally induced denaturation in the absence and presence of Ficoll 70. Figure 2 also shows that higher concentration Ficoll 70 can retard the thermally induced unfolding of CK. This effect is dependent on the concentration of Ficoll 70.Fig. 2. Temperature dependence of the unfolding fraction (fu) of CK calculated according to Eq. (4). The Ficoll 70 concentrations (mg/ml) were 0, 30, 60, 90, 120, 180, 240, and 260 (curves 1-8), respectively.
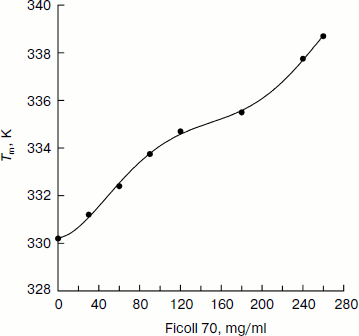
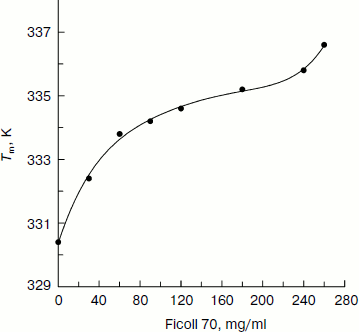
The best-fit values of the thermally induced unfolding parameters of CK are summarized in the table. The plot of the transition temperature (Tm) of CK as a function of Ficoll 70 concentration (Fig. 3) shows that Tm increases by up to 8 K with increasing Ficoll 70 concentration from 0 to 260 mg/ml, suggesting higher concentration of Ficoll 70 can significantly increase the thermal stability of CK secondary structure, thus retarding the thermally induced unfolding of CK. In addition, it can also be seen in the table that the values of ΔHu change only a little with the increase in Ficoll 70 concentration. No systematic dependence of this value on either Tm or Ficoll 70 concentration is observable.
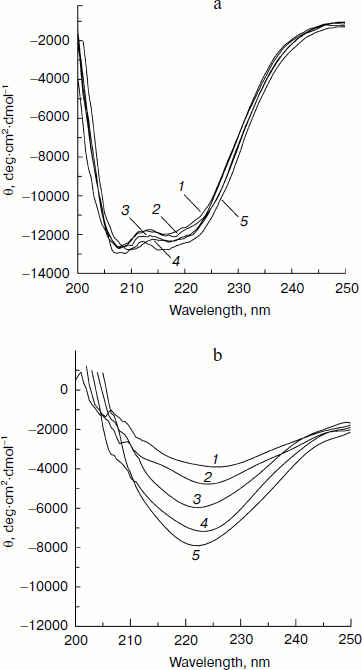
Figure 4 (a and b) shows the far-UV CD spectra of native CK (at 298 K) and thermally denatured CK (at 353 K) in the presence of different concentrations of Ficoll 70 (0-240 mg/ml). The results show that the ellipticity of both thermally denatured and native CK increases with increasing Ficoll 70 concentration, suggesting gain of secondary structure as a function of added crowding agent. The ellipticity at 222 nm for thermally denatured CK increased by 112% in the presence of 240 mg/ml Ficoll 70 compared to that in the absence of Ficoll 70, while the ellipticity only increased by 8.4% for native CK, showing that the effect is much more significant for thermally denatured CK than it is for native CK.Fig. 3. Transition temperature (Tm) of thermally induced denaturation of CK as a function of Ficoll 70 concentrations based on far-UV CD measurements.
Measurement and analysis of fluorescence spectra for effect of Ficoll 70 on CK. Thermally induced denaturation of CK was also studied by tryptophan fluorescence spectra. The red-shift of λmax was used to calculate the unfolding fraction (fu) of CK at different temperature in the absence and presence of Ficoll 70. The unfolding process was assumed to follow a two-state transition between native and denatured states, as discussed above. The value of fu was assumed to be 0% at 298 K and 100% at 353 K for all Ficoll 70 concentrations separately.Fig. 4. Far-UV CD spectra of (a) native (at 298 K) and (b) thermally induced unfolded (at 353 K) CK in the presence of various amounts of Ficoll 70. The concentrations of Ficoll 70 for curves 1-5 are 0, 60, 120, 180, and 240 mg/ml, respectively.
fu = [λmax(T) − λmax(298 K)]/[λmax(353 K) − λmax(298 K)] (5)
The value of fu calculated according to Eq. (5) is plotted versus temperature in Fig. 5. The transition curves in Fig. 5 indicate that the unfolding of the CK tertiary structures follows a two-state cooperative process in the absence and presence of Ficoll 70, which is in good agreement with the observation from CD spectra.
Additionally, it also shows that Ficoll 70 has an obvious protective effect against thermally induced unfolding of CK, and this effect is dependent on Ficoll 70 concentration. Figure 6 shows the value of Tm monitored by fluorescence spectra increases from 330.4 to 336.6 K with Ficoll 70 concentration increasing from 0 to 260 mg/ml, which is in good accordance with the calculated value based on CD spectra. The plot of Tm as a function of the concentration of Ficoll 70 is similar to Fig. 3.Fig. 5. Temperature dependence of the unfolded fraction (fu) of CK calculated according to Eq. (5). The Ficoll 70 concentrations (mg/ml) were 0, 30, 60, 90, 120, 180, 240, and 260 (curves 1-8), respectively.
Fig. 6. Transition temperature (Tm) of thermally induced denaturation of CK as a function of Ficoll 70 concentrations based on fluorescence measurement.
DISCUSSION
This paper has addressed the general issue of effect on protein stability by volume exclusion in a biological environment, otherwise known as “macromolecular crowding” [12, 29-33]. Specifically, we chose a dimeric enzyme with relatively complex structure and significant biological function as the model protein. In contrast, previous studies usually focused on small proteins or peptides that have a simple folding mechanism.
The study in this paper analyzed the changes of secondary structure and tertiary structure of CK from 298 to 353 K in the presence of various concentrations of Ficoll 70. The equilibrium unfolding of CK in the presence of Ficoll 70 is highly cooperative, as judged by the quality of the two-state model fitting to individual transitions and the coincidence of transition curves monitored by far-UV CD spectra and fluorescence spectra.
The most favorable situation for evaluating the molecular crowding effect on protein stability occurs when the equilibrium between native and unfolded states is a two-state process devoid of protein–protein associations. As CK is a dimer protein, the influence of the association between two monomers on unfolding equilibrium should be considered. Early studies have shown that the self-association of protein molecules might contribute to the increase in Tm with increasing crowding agent concentration if macromolecular crowding agent greatly affects protein–protein association reactions [7]. This means the increase in protein concentration will bring about an increase in Tm in the presence of crowding agent. However, our data reveal that the value of Tm in the presence of 260 mg/ml Ficoll 70 does not change with increase in protein concentration from 0.12 to 0.3 mg/ml, suggesting that Ficoll 70 has no obvious effect on interaction between CK monomers and significant protein association does not occur under the defined experimental conditions (table).
Thermodynamic parameters of thermally induced denaturation of CK in the
presence of Ficoll 70
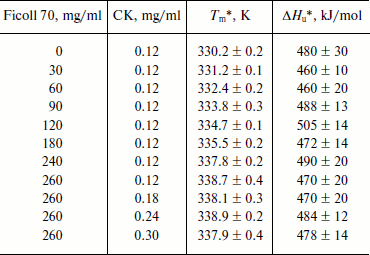
* Tm and ΔHu values
at each given concentration of Ficoll 70 and CK are calculated using a
two-state model based on Eqs. (1)-(3) in the text. These values are
expressed as the means ± S.D. to denote the uncertainties from
quality of fit.
Our results provide additional strong evidence that macromolecular crowding enhances protein stability. Enhanced thermal stabilities were observed by monitoring the changes in secondary structure and tertiary structure of CK. The transition temperature, Tm, of a thermally induced transition curve for a protein is one of the most commonly used indices of protein thermostability [34]. All our results, including CD spectra and fluorescence spectra, showed that Ficoll 70 markedly increases the Tm value of CK during thermally induced unfolding, indicating that Ficoll 70 has a clear protective effect against thermally induced unfolding of CK.
The equilibrium statistical–thermodynamic model for the effect of volume exclusion arising from high concentrations of stable macromolecules upon the stability of trace globular proteins, developed primarily by Minton and coworkers, predict that macromolecules will favor globular conformations as the extent of macromolecular crowding is increased. Thermodynamic calculations using the principles of excluded volume theory show that the introduction of sufficiently large concentrations of stable macromolecules of molar mass comparable to that of a labile trace protein can reduce the equilibrium constant (K) for denaturation of the labile protein by one or two orders of magnitude. This model was then elaborated to estimate the magnitude of crowding effects on the thermal denaturation of proteins. As a result, this application predicts an increase in Tm at physiological solute concentrations in a range of 5-20°C [15]. In our experiments, the data agree qualitatively with this prediction and appear to provide additional support for the model of molecular crowding based on excluded volume theory.
The data in the table show that ΔHu is independent of Ficoll 70 concentration. To the best of our knowledge, the stability of a compact native structure of a protein in aqueous solution is determined by the interactions among the groups of protein molecules and the surrounding water molecules [35]. Macromolecular crowding would favor any state of the system that excludes the smallest volume from the highly concentrated background molecules based on the previous viewpoint. The crowding effect reduces the configurational entropy of the unfolded state through the steric excluded volume effect [14, 15]. Therefore, if a crowding agent exhibits little enthalpic interaction with the reacting protein, crowding theory would predict an increase in Tm value with constant ΔHu in the thermally induced unfolding with increasing concentration of crowding macromolecules. This theoretical prediction is consistent with our observation: a noticeable increase in Tm value, while the value of ΔHu is almost constant with increasing Ficoll 70 concentration.
The excluded-volume theory implies that macromolecular crowding acts mainly on unfolded-state conformations. By making the denatured state more compact and thereby less energetically favorable, the native state is indirectly stabilized [12, 36-38]. The statistical–thermodynamic model of Minton also predicts that excluded volume in concentrated solutions of stable “inert” macrosolutes can serve to stabilize the globular native state of proteins against unfolding by preferentially destabilizing the unfolded state. The model further predicts that the average dimension of the unfolded state will decrease with increasing concentration of inert macrosolute [15]. In our experiments, we observed a compaction of the structure of both native protein and thermally denatured protein (i.e. more ellipticity) of CK in the presence of crowding macromolecules. The structural effect appears more obvious on the denatured than the folded ensemble. Our experimental observations on the structural changes in both native and denatured states of CK also indicate that the crowding effects on denatured protein molecules are feasible, which agrees qualitatively with the prediction of the excluded volume theory.
In summary, we have observed the influence of macromolecular crowding agent Ficoll 70 on the structure and stability of CK during thermally induced unfolding. The general enhancement of thermal stability of CK in the presence of Ficoll 70 provides strong support to the theory-based models of macromolecular crowding. The data presented here perhaps will motivate the development of more realistic models for crowding macromolecules and their influences on the folded and unfolded states of proteins. Also, our findings imply that the effects of macromolecular crowding can be an important part of understanding how protein folding occurs in a very complex intracellular environment.
This work was supported by grants of the National Natural Science Foundation of China (No. 30800188) and the Scientific Research Foundation for Returned Overseas Chinese Scholars, State Education Ministry.
REFERENCES
1.Anfinsen, C. B. (1973) Science, 181,
223-230.
2.Fulton, A. B. (1982) Cell, 30,
345-347.
3.Zimmerman, S. B., and Trach, S. O. (1991) J.
Mol. Biol., 222, 599-620.
4.Ellis, R. J., and Minton, A. P. (2003)
Nature, 425, 27-28.
5.Medalia, O., Weber, I., Frangakis, A. S., Nicastro,
D., Gerisch, G., and Baumeister, W. (2002) Science, 298,
1209-1213.
6.Chebotareva, N. A., Kurganov, B. I., and Livanova,
N. B. (2004) Biochemistry (Moscow), 69, 1239-1251.
7.Van den Berg, B., Ellis, R. J., and Dobson, C. M.
(1999) EMBO J., 18, 6927-6933.
8.Van den Berg, B., Wain, R., Dobson, C. M., and
Ellis, R. J. (2000) EMBO J., 19, 3870-3875.
9.Uversky, V. N., Coopera, E. M., Bower, K. S., Li,
J., and Fink, A. L. (2002) FEBS Lett., 515, 99-103.
10.Cheung, M. S., Klimov, D., and Thirumalai, D.
(2005) Proc. Natl. Acad. Sci. USA, 102, 4753-4758.
11.Ai, X., Zhou, Z., Bai, Y., and Choy, W. Y. (2006)
J. Am. Chem. Soc., 128, 3916-3917.
12.Zimmerman, S. B., and Minton, A. P. (1993)
Anne. Rev. Biophys. Biomol. Struct., 22, 27-65.
13.Minton, A. P. (2000) Curr. Opin. Struct.
Biol., 10, 34-39.
14.Minton, A. P. (2001) J. Biol. Chem.,
276, 10577-10580.
15.Minton, A. P. (2000) Biophys. J.,
78, 101-109.
16.Tellam, R. L., Sculley, M. J., Nichol, L. W., and
Wills, P. R. (1983) Biochem J., 213, 651-659.
17.Sasahara, K., McPhie, P., and Minton, A. P.
(2003) J. Mol. Biol., 326, 1227-1237.
18.Stagg, L., Zhang, S. Q., Cheung, M. S., and
Wittung-Stafshede, P. (2007) Proc. Natl. Acad. Sci. USA,
104, 18976-18981.
19.Watts, D. C. (1973) in The Enzymes (Boyer,
P. D., ed.) 3rd Edn., Vol. 8, Academic Press, New York, pp.
383-455.
20.He, H. W., Zhang, J., Zhou, H. M., and Yan, Y. B.
(2005) Biophys. J., 89, 2650-2658.
21.Zhou, H. M., and Tsou, C. L. (1986) Biochim.
Biophys. Acta, 869, 69-74.
22.Zhou, H. M., Zhang, X. H., Yin, Y., and Tsou, C.
L. (1993) Biochem. J., 291, 103-107.
23.Yang, Y., and Zhou, H. M. (1998) Biochim.
Biophys. Acta - Protein Struct. Mol. Enzymol., 1388,
190-198.
24.Yao, Q. Z., Zhou, H. M., Hou, L. X., and Tsou, C.
L. (1982) Sci. Sin. (Ser. B), 25, 1296-1302.
25.Yao, Q. Z., Tian, M., and Tsou, C. L. (1984)
Biochemistry, 23, 2740-2744.
26.Zhou, H. X., and Dill, A. (2001)
Biochemistry, 40, 11289-11293.
27.Eftink, M. R., and Ramsay, G. D. (1994) Meth.
Enzymol., 240, 615-645.
28.Becktel, W. J., and Schellman, J. A. (1987)
Biopolymers, 26, 1859-1877.
29.Hall, D., and Minton, A. P. (2003) Biochim.
Biophys. Acta, 107, 299-316.
30.Ellis, R. J. (2001) Trends Biochem. Sci.,
26, 597-604.
31.Hall, D., and Dobson, C. M. (2006) FEBS
Lett., 580, 2584-2590.
32.Spencer, D. S., Xu, K., Logan, T. M., and Zhou,
H. X. (2005) J. Mol. Biol., 351, 219-232.
33.Eggers, D. K., and Valentine, J. S. (2001)
Protein Sci., 10, 250-261.
34.Karantzeni, I., Ruiz, C., Liu, C. C., and Licata,
V. J. (2003) Biochem. J., 374, 785-792.
35.Makhatadze, G. I., and Privalov, P. L. (1995)
Adv. Protein Chem., 47, 307-425.
36.Minton, A. P. (2005) Biophys. J.,
88, 971-985.
37.Minton, A. P. (2005) J. Pharmacol. Sci.,
94, 1668-1675.
38.Zhou, H. X. (2004) J. Mol. Recognit.,
17, 368-375.