Discovery of a Photosynthesizing Animal that Can Survive for Months in a Light-Dependent Manner
V. P. Skulachev
Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: (495) 939-0338; E-mail: skulach@belozersky.msu.ru
Received September 14, 2010
Recently M. E. Rumpho and coworkers (USA) established that the marine slug Elysia chlorotica, a gastropod mollusk that feeds on the eukaryotic filamentous yellow-green alga Vaucheria litorea, recruits chloroplasts from the alga and transports them from the digestive apparatus into a special organ of the slug that resembles a green leaf and is an approximately 100-fold increased parapodium—an outgrowth of the slug’s body. The chloroplasts survive inside the slug for up to 10 months and perform active photosynthesis accompanied by assimilation of CO2. Under conditions of starvation, this photosynthesis becomes for the animal the only source of energy and fixed carbon. For functioning, chloroplasts have to constantly import some short-lived proteins that are encoded in the nucleus of the photosynthesizing organism. Therefore, the authors supposed that a transfer of the corresponding genes must have occurred between the algal and mollusk nuclei. This hypothesis was experimentally confirmed for two genes encoding proteins of the photosynthesizing apparatus. The questions arise of what mechanism was responsible for the transfer of these genes and how the slug created its photosynthesizing organ resembling the leaf of a higher plant rather than the primitive filamentous algal structure which was the source of the acquired chloroplasts and the photosynthesis genes.
KEY WORDS: photosynthesis, animal, mollusk, chloroplastDOI: 10.1134/S0006297910120114
American biochemists under the guidance of Mary E. Rumpho made an amazing discovery. They showed [1-4] that the small marine slug Elysia chlorotica, which feeds on the photosynthesizing filamentous yellow-green alga Vaucheria litorea, while digesting the alga collects its chloroplasts and transfers them into a special photosynthesizing organ resembling a green leaf. After collecting the chloroplasts and including them into its leaf-like organ, the slugs can live on the energy of light, not needing the algae during its further life, i.e. up to 10 months. The authors demonstrated the incorporation in the light of labeled CO2 into the mollusk’s carbohydrates by means of the Calvin cycle enzymes which are absent in other animals. At least two genes of chloroplast proteins localized in the cell nucleus of the alga were transferred into the cell nucleus of the mollusk. These are the gene psbO encoding the protein MSP which stabilizes the manganese complex of the photosystem II [3] and the gene of phosphoribulose kinase of the Calvin cycle [4]. In both cases the genes were shown to be incorporated into nuclei of the embryonic line cells of the gastropod. The works of Rumpho and colleagues are performed using the most modern approaches of molecular biology, and there is no doubt of their reliability.
Chloroplasts are currently thought to contain from 2000 to 4000 different proteins, and no more than 10% of them are encoded by DNA of chloroplasts. Comparison of these numbers allows us to imagine the scale of possible transfer of genes from the nuclei of the alga into the nuclei of the mollusk. Note that chloroplasts of V. litorea are discriminated from similar organelles of green algae and higher green plants by significantly greater stability. Thus, chloroplasts of spinach retain their activity in vitro for a few hours after isolation, whereas isolated chloroplasts of V. litorea are active during three days [5, 6]. But it is unlikely that even the more stable chloroplasts can survive for months without import of proteins encoded in the cell nucleus. The matter is that many proteins of chloroplasts are short-lived because they are inactivated by reactive oxygen species [7].
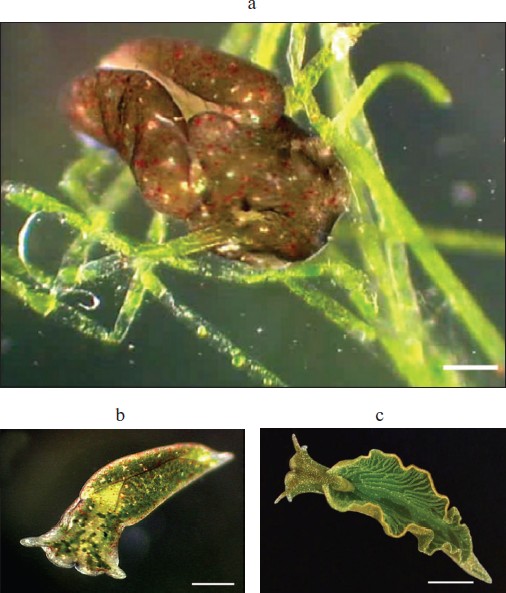
The mollusk’s chloroplasts “stolen” from the alga were wittily called “cleptoplasts”. The “cleptoplasts” are encircled by a typical chloroplast envelope consisting of two membranes, and one can see thylakoids inside the organelles. It is interesting that envelop of chloroplasts of V. litorea consists of four membranes, whereas that of chloroplasts of higher green plants consists of two membranes—chloroplasts of V. litorea are additionally surrounded by two membranes of endoplasmic reticulum1. But the difference in the localization of chloroplasts in the alga and in the mollusk is more striking. The alga does not have a special organ responsible for photosynthesis and the chloroplasts are equally distributed inside long tube-shaped cells. The mollusk presents another picture. Once having eaten the algae, it increases greatly in size and step-by-step forms the earlier absent photosynthesizing organ, which has a shape of a higher plant leaf. And just inside this organ the majority of “stolen” chloroplasts are placed. Certainly, it could be supposed that this effect is a result of the convergent evolution of higher plants and of a mollusk that has acquired cleptoplasts. But it is believed that evolution of algae to higher plants has taken millions of years. And the reasonable question is what a genial engineer has helped a mollusk to traverse such a rather long pathway. Of course, the authors of the discovery have not decided to ask this sacramental question in their publications and do not even mention the strikingly obvious resemblance of the green leaf and the mollusk who had acquired photosynthesis (figure; see color insert).
1Chloroplasts of yellow-green algae are really results of a relatively recent endosymbiosis of a non-phototrophic organism with a photosynthesizing alga. This explains their encirclement by membranes of endoplasmic reticulum.
In a letter received from M. E. Rumpho after submission of this note to the journal it is stated that complete sequence of the mollusk chromosomal DNA did not reveal the genes of photosynthetic proteins. This fact can be explained if such genes are localized in non-chromosomal DNA of the animal (M. E. Rumpho, personal communication, 2010).

One could possibly consider the above-presented data as a very refined joke by Mary Rumpho and her thirteen coauthors from eight different laboratories from seven American cities and one Korean laboratory. But publications by Rumpho from 2000 appeared in the respectable international journals under reviews (one of the latest publications appeared in P.N.A.S.), and the phenomenon of chloroplast presence inside a leaf-like organ of the mollusk E. chlorotica is known from 1979 from the description by Graves et al. [8] and by West [9]. Moreover, five years before the “cleptoplasty” phenomenon was described on other objects by Hinde and Smith [10]. (For more recent works on this theme see publications by Raven [11] and Gustafson et al. [12]).
REFERENCES
1.Rumpho, M. E., Summer, E. J., and Manhart, J. R.
(2000) Plant Physiol., 123, 29-38.
2.Rumpho, M. E., Summer, E. J., Green, B. J., Fox, T.
C., and Manhart, J. R. (2001) Zoology (Jena), 104,
303-312.
3.Rumpho, M. E., Worful, J. M., Lee, J., Kannan, K.,
Tyler, M. S., Bhattacharya, D., Moustafa, A., and Manhart, J. R. (2008)
Proc. Natl. Acad. Sci. USA, 105, 17867-17871.
4.Rumpho, M. E., Pochareddy, S., Worful, J. M.,
Summer, E. J., Bhattacharya, D., Pelletreau, K. N., Tyler, M. S., Lee,
J., Manhart, J. R., and Soule, K. M. (2009) Mol. Plant,
2, 1384-1396.
5.Walker, D. A., Baldry, C. W., and Cockburn, W.
(1968) Plant Physiol., 43, 1419-1422.
6.Green, B. J., Li, W. Y., Manhart, J. R., Fox, T.
C., Summer, E. J., Kennedy, R. A., Pierce, S. K., and Rumpho, M. E.
(2000) Plant Physiol., 124, 331-342.
7.Mattoo, A. K., Hoffman-Falk, H., Marder, J. B., and
Edelman, M. (1984) Proc. Natl. Acad. Sci. USA, 81,
1380-1384.
8.Graves, D. A., Gibson, M. A., and Bleakney, J. S.
(1979) Veliger, 21, 415-422.
9.West, H. H. (1979) Chloroplast Symbiosis and
Development of the Ascoglossum Opisthobranch Elysia chlorotica: PhD
Thesis, Northwestern University.
10.Hinde, R., and Smith, D. C. (1974) Biol. J.
Linn. Soc., 6, 349-356.
11.Raven, J. A. (1997) Luminol. Oceanogr.,
42, 198-205.
12.Gustafson, D. E., Jr., Stoecker, D. K., Johnson,
M. D., van Heukelem, W. F., and Sneider, K. (2000) Nature,
405, 1049-1052.