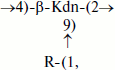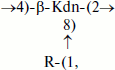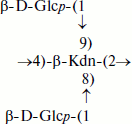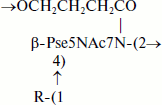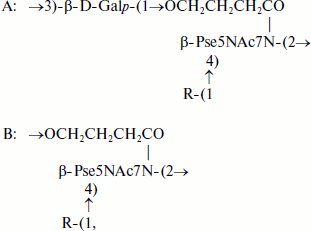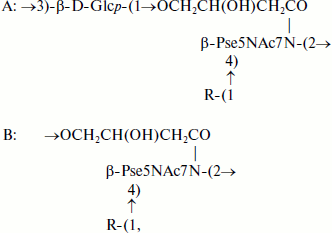REVIEW: Teichuronic and Teichulosonic Acids of Actinomycetes*
E. M. Tul’skaya1**, A. S. Shashkov2, G. M. Streshinskaya1, S. N. Senchenkova2, N. V. Potekhina1, Yu. I. Kozlova1, and L. I. Evtushenko3
1Biological Faculty, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: (495) 939-4309; E-mail: em_tulskaya@mail.ru2Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky pr. 47, 119991 Moscow, Russia; fax: (499) 135-5328
3All-Russian Collection of Microorganisms (VKM), Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, pr. Nauki 5, 142290 Pushchino, Moscow Region, Russia; fax: (495) 956-3370
* This review is based on a presentation made at the 4th Baltic Conference on Microbial Carbohydrates, Hyytiala Forestry Field Station, Finland, September 19-22, 2010.
** To whom correspondence should be addressed.
Received January 25, 2011; Revision received February 10, 2011
The subject of the present review is the structural diversity and abundance of cell wall teichuronic and teichulosonic acids of representatives of the order Actinomycetales. Recently found teichulosonic acids are a new class of natural glycopolymers with ald-2-ulosonic acid residues: Kdn (3-deoxy-D-glycero-D-galacto-non-2-ulosonic acid) or di-N-acyl derivatives of Pse (5,7-diamino-3,5,7,9-tetradeoxy-L-glycero-L-manno-non-2-ulosonic or pseudaminic acid) as the obligatory component. The structures of teichuronic and teichulosonic acids are presented. Data are summarized on the occurrence of the glycopolymers of different nature in the cell wall of the studied actinomycetes. The biological role of the glycopolymers and their possible taxonomic implication are discussed. The comprehensive tables given in the Supplement show 13C NMR spectroscopic data of teichuronic and teichulosonic acids obtained by the authors.
KEY WORDS: cell wall, actinomycetes, teichuronic acid, teichulosonic acid, NMR spectroscopyDOI: 10.1134/S0006297911070030
Abbreviations: Gal2,3OMe, 2,3-di-O-methylgalactose; Gal3OMe, 3-O-methylgalactose (madurose); GlcNAc3NAc, 2,3-diacetamido-2,3-dideoxyglucose; GlcNAc3NAcA, 2,3-diacetamido-2,3-dideoxyglucuronic acid; Glu, glutamic acid; Kdn, 3-deoxy-D-glycero-D-galacto-non-2-ulosonic acid; ManNAcA, 2-acetamido-2-deoxymannuronic acid; ManNAc3NAcA, 2,3-diacetamido-2,3-dideoxymannuronic acid; NP, neutral polysaccharide; PGP, poly(glycosyl 1-phosphate); Pse, 5,7-diamino-3,5,7,9-tetradeoxy-L-glycero-L-manno-non-2-ulosonic (pseudaminic) acid; Pyr, pyruvic acid; Rha, rhamnose; TA, teichoic acid; TUA, teichuronic acid; TULA, teichulosonic acid.
Thirteen years have elapsed since the publication of a review [1] devoted to structures of cell wall anionic polymers
of Gram-positive bacteria. Over the past decade, considerable progress
has been made in this area: cell walls of more than hundred bacterial
strains belonging to different genera and species have been
investigated and anionic polymers with unique structures discovered,
including those characteristic of Gram-positive bacteria only. The
notion on the abundance of different polymers in cell walls of these
organisms has changed. Thus from the analysis of data presented in
review [1], it could be inferred that the negative
charge of the cell wall is usually (in >90% strains studied) due to
the presence of phosphoric acid residues contained in teichoic acids
(τοιχος – wall), such as
poly(polyol phosphates) and poly(glycosylpolyol phosphates), as well as
poly(glycosyl 1-phosphates). A fairly rare exception was a family of
teichuronic acids, which impart the negative charge to the cell wall
due to the constituent uronic acids. Recent studies have demonstrated
that teichuronic acids are rather common cell wall components of
actinomycetes. Moreover, other anionic polymers (teichulosonic acids)
have been found, where it is ald-2-ulosonic acids that are responsible
for their negative charge. As in the case of teichoic acids, the
presence and structural features of teichuronic and teichulosonic acids
can serve as chemical markers for taxonomy of actinomycetes. Novel
teichuronic and teichulosonic acids with hitherto unknown structures
discovered in cell walls of actinomycetes are quite numerous, and the
present review is devoted to the authors’ experimental data only
of these compounds that were reported over the period from 1994 to
2011.
TEICHURONIC ACIDS
Teichuronic acids are natural biopolymers known for about 50 years. Their indispensable components are uronic acids, which, together with amino sugars and neutral monosaccharides, are linked to a polymer by glycosidic bonds. Sometimes these polymers contain residues of organic acids [2, 3] including amino acids [4, 5] as well as glycerol phosphate [6]. Teichuronic acids are localized in the cell walls of Gram-positive bacteria and are covalently linked to peptidoglycan [1, 7].
The term “teichuronic acids” was first proposed for a polysaccharide isolated from the cell wall of Bacillus licheniformis NCTC 6346 [8]. Its repeating unit comprised glucuronic acid and N-acetylglucosamine. To date, teichuronic acids have been found in representatives of the genera Bacillus, Micrococcus, Streptococcus, Staphylococcus, and several genera of the order Actinomycetales, viz. Propionibacterium, Corynebacterium, Catellatospora [1, 7], Actinoplanes, Streptomyces [5, 9-13], and Kribbella [14]. However, complete structures of teichuronic acids are far from being established for all of them, and quite often only the composition of the glycopolymers has been reported [1].
For several bacilli, it was demonstrated that incorporation of teichuronic acids in the cell wall occurs only on culturing on a medium with deficiency of phosphate, and they are not synthesized on complete media [15]. However, teichuronic acids were found in cell walls of actinomycetes cultured on media balanced in phosphorus content.
In some cases teichuronic acids are the only cell wall anionic polymers of Gram-positive bacteria, as exemplified by Bacillus megaterium M46 [16]. However, cell walls of certain bacteria, e.g. B. licheniformis ATCC 9945 [17], contain both teichoic and teichuronic acids. Actinomycetes studied comprise, in addition to teichuronic acids, other polymers, viz. teichoic and teichulosonic acids as well as neutral polysaccharides (table). It was established that teichoic and teichuronic acids of B. licheniformis NTCT 6346 are linked to different glycan chains of peptidoglycan [18], which can imply similar location of other glycopolymers when present simultaneously in the cell wall of a microorganism [19].
Cell wall glycopolymers of representatives of the order Actinomycetales
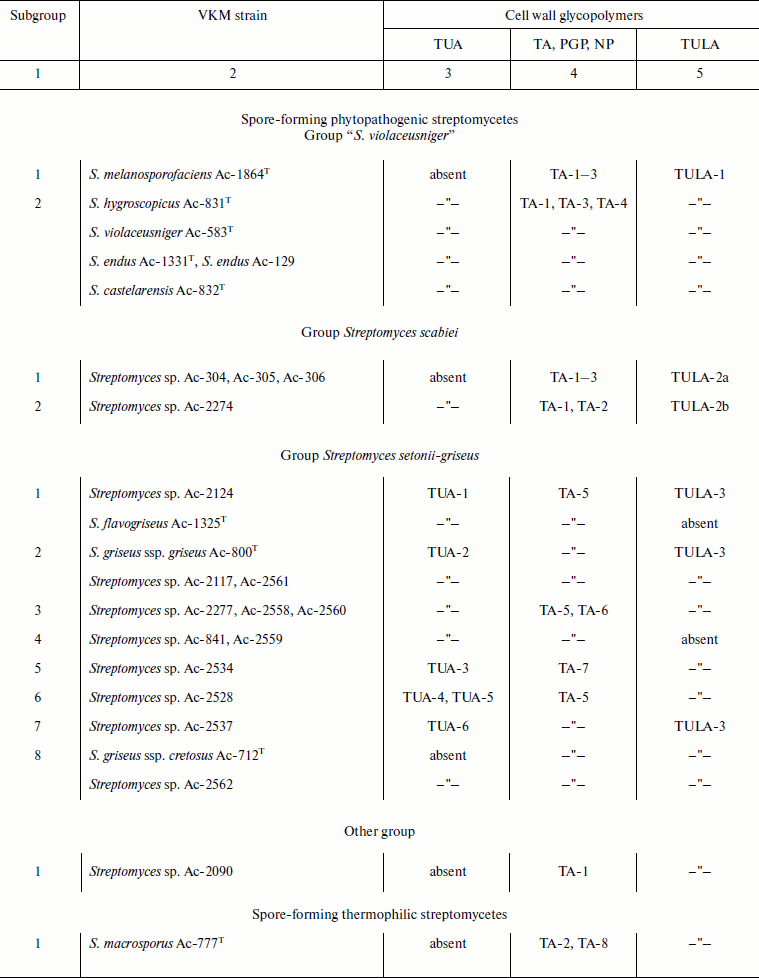
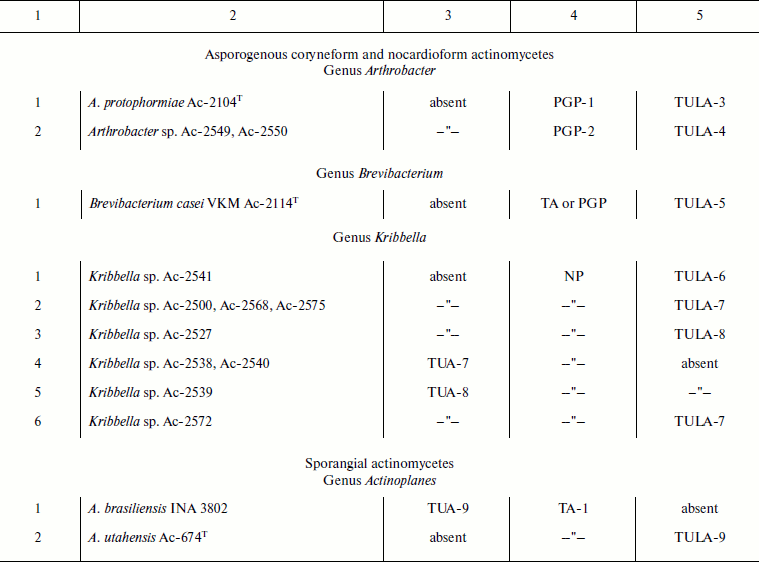
Notes: VKM, All-Russian Collection of Microorganisms; PGP, poly(glycosyl
1-phosphate); NP, neutral polysaccharide; TA, teichoic acid; TUA,
teichuronic acid; TULA, teichulosonic acid; TA-1, 1,3-poly(glycerol
phosphate) (1,3-poly(GroP)); TA-2, 1,3-poly(GroP) bearing
α-D-GlcpNAc and L-Lys [30]; TA-3,
2,3-poly(GroP); TA-4, 1,3-poly(GroP) bearing α-D-GlcpN and
α-D-GlcpNAc as well as L-Lys [51];
TA-5, 1,5-poly(ribitol phosphate) (1,5-poly(RboP)) bearing
β-D-Glcp; TA-6, 1,5-poly(RboP) bearing Pyr; TA-7,
1,5-poly(RboP) bearing β-D-Glcp and/or
α-D-GlcpNAc; TA-8,
-6)-β-D-Galp-(1→1)-Gro-(3-P-; PGP-1,
→6)-α-D-GlcpNAc-(1-P-6)-α-D-GalpNAc-(1→
[31]; PGP-2,
→3)-β-D-Galp-(1-P-6)-β-D-GalpNAc-(1→
[31]; NP,
→6)-[α-D-Manp-(1→2)]-α-D-Manp-(1→
[14].
Wide structural diversity of teichuronic acids is determined by (i) the nature of uronic acids (glucuronic and mannuronic acids and their mono- and diamino derivatives) and other monosaccharides (glucose and its mono- and diamino derivatives, galactose and its amino derivatives, rhamnose, sometimes fucose, and 6-deoxytalose); (ii) the presence of different N-acyl and other non-sugar substituents; (iii) the number of monomers in the repeating unit and the number of the units in the polymer chain; (iv) the topology of the polymer, i.e. its linear or branched structure; (v) the position and configuration of the glycosidic bonds.
Over the last several years, teichuronic acids have been detected and characterized in the cell walls of actinomycetes. In all cases, they were accompanied by other glycopolymers (table). Spectroscopic characteristics of the identified teichuronic acids are listed in Supplement Table 1 (available on the site of Biochemistry (Moscow), http://protein.bio.msu.ru/biokhimiya).
Teichuronic acids with linear disaccharide repeating units. Fifteen strains of phytopathogenic streptomycetes that cause potato and root scab and form a phylogenetic branch of Streptomyces setonii-griseus were studied [5, 10-13], and the following structures of teichuronic acids with linear disaccharide repeating units were established in 13 of them:
TUA 1 →4)-β-D-ManpNAc3NAcA-(1→6)-α-D-Glcp-(1→ [10];
TUA 2 →4)-β-D-ManpNAc3NAcA-(1→3)-α-D-GalpNAc-(1→ [11, 12];
TUA 3 →4)-β-D-ManpNAc3NAcylA-(1→3)-α-D-GalpNAc-(1→, where Acyl is Ac or L-Glu [5];
TUA 4 →4)-β-D-ManpNAc3NAcA-(1→6)-α-D-GlcpNAc-(1→ [13];
TUA 5 →4)-β-D-GlcpNAc3NAcA-(1→6)-α-D-GlcpNAc-(1→ [13];
TUA 6 →4)-β-D-GlcpNAc3NAcA-(1→3)-α-D-GalpNAc-(1→ [13].
Teichuronic acids 1-4 contain diaminomannuronic acid, whereas TUA 5 and 6 include diaminoglucuronic acid. In all polymers, the acidic monomers are substituted at position 4 and attached to α-linked glucose or an amino sugar by β-(1→3)- or β-(1→6)-glycosidic bond.
A characteristic feature of cell wall TUA 3 from Streptomyces sp. VKM Ac-2534 is the presence of L-glutamic acid as the 3-N-acyl substituent of diaminomannuronic acid [5]. Earlier, teichuronic acids from B. subtilis AHU 1219 have been shown to contain N-linked serine and threonine [4].
Cell wall teichuronic acids with similar structures have been found in representatives of the genus Kribbella:
TUA 7 →4)-β-D-ManpNAc3NAcA-(1→6)-α-D-GlcpNAc3NAc-(1→ [14];
TUA 8 →4)-β-D-ManpNAcA-(1→6)-α-D-GlcpNAc3NAc4OAc-(1→ [14, 20].
The feature of these teichuronic acids is the presence of 2,3-diacetamido-2,3-dideoxyglucose, an uncommon polymer constituent, or its 4-O-acetyl derivative. The polymer chains of TUA-1–8 consist of 8-15 repeating units.
It should be noted that teichuronic acids with linear disaccharide repeating units are the most common polymers of this class; earlier, they have been reported in representatives of the genera Bacillus [8, 21] and Micrococcus [22-24] and in strain Staphylococcus aureus T [25]. Their structures can be found in review [1].
Teichuronic acids with four and more monosaccharides in the repeating unit. The first actinomycete where the cell wall teichuronic acid was found was Actinoplanes brasiliensis INA 3802 [9]. This polymer (TUA 9) comprises six repeating units, which are represented by a linear tetrasaccharide containing glucose, diaminoglucose, diaminoglucuronic, and diaminomannuronic acids and has the following structure: →6)-α-D-Glcp-(1→4)-β-D-GlcpNAc3NAcA-(1→4)-α-D-GlcpNAc3NAc-(1→4)-β-D-ManpNAc3NAcA-(1→.
Earlier, teichuronic acids with linear tetrasaccharide repeating units have been identified in cell walls of B. subtilis AHU 1031 [6] and B. licheniformis ATCC 9945 [17]. Polymers with more complex structures are also known. Thus the repeating units of cell wall teichuronic acids of B. subtilis AHU 1031 [6] and Propionibacterium acnes C7 [26] contained five and more monosaccharides. Their structures are shown in review [1].
A novel unique structure of cell wall teichuronic acid of Geobacillus stearothermophilus PV72/p2 has recently been described [3]. The branched polymer contained N-acetylglucosamine, N-acetylmannosamine, aminomannuronic acid and pyruvic acid (structural formula (1)).
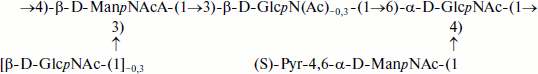 (1)
(1)Therefore, the structures of teichuronic acids of actinomycetes are quite diverse. Seven different monosaccharide components have been identified, and the repeating units comprise from two to four and more monosaccharide residues linked by 1→3-, 1→4-, or 1→6-glycosidic bonds with β- or α-configuration of the glycosidic centers. Uronic acids, chiefly mannuronic acid, are represented by amino and diamino derivatives. In addition, O-acetyl groups and N-linked L-glutamic acid have been detected.
TEICHULOSONIC ACIDS
The term “teichulosonic acids” was proposed by Yu. A. Knirel in 2009 [27] to denote glycopolymers of cell walls of Gram-positive bacteria containing ald-2-ulosonic acids as the indispensable constituent.
Teichulosonic acids were discovered about a decade ago in cell walls of phytopathogenic streptomycetes that cause potato and root scab. They contained 3-deoxy-D-glycero-D-galacto-non-2-ulosonic acid (Kdn) as a monosaccharide constituent [10-13, 28-30]. Recently, cell walls of other representatives of the order Actinomycetales (Kribbella and Actinoplanes) were shown to comprise N,N′-diacyl derivatives of 5,7-diamino-3,5,7,9-tetradeoxy-L-glycero-L-manno-non-2-ulosonic acid (pseudaminic acid, Pse) [14, 20]. Thus two types of teichulosonic acids are known to date, viz. glycopolymers containing Kdn or Pse residues.
Teichulosonic acids containing Kdn residues. The basic chain of this type of teichulosonic acids is composed of α- or β-(2→4)-linked Kdn residues, which bear glucopyranose, galactopyranose, 3-O-methylgalactopyranose, or N-acetylglucosamine substituents at O8 and/or O9. Both Kdn oligomers [29] and polymers [10-13, 28, 30-32] are known.
Currently, Kdn-containing teichulosonic acids have been found in the cell walls of phytopathogenic and thermophilic streptomycetes as well as asporogenous coryneform actinomycetes along with other glycopolymers (table). The spectroscopic characteristics of these polymers are given in Supplement Table 2.
Teichulosonic acid 1 was found among cell wall polymers of six strains of the phylogenetic group “S. violaceusniger” [30], and teichulosonic acid 2a was detected in the cell walls of four strains of the phylogenetic group S. scabiei [13]. These glycopolymers possessed similar structures, the β-Kdn residues being glycosylated at O9 with β-D-Galp residues. In teichulosonic acid 2a, some β-D-Galp residues are 3-O-methylated:
where R = β-D-Galp (TULA 1) or R = β-D-Galp or β-D-Galp3OMe (TULA 2a).
Teichulosonic acid 2b isolated from the cell wall of a phytopathogenic streptomycete Streptomyces sp. VKM Ac-2274 [29] is a tetrasaccharide with two Kdn residues, which are α-(2→4)-interlinked and substituted at O9 with β-D-Galp and/or β-D-Galp3OMe in molar ratio ~2 : 1 (see formula (2)). The hydroxyl groups at C7 and C8 of the Kdn residues and at C2 of galactopyranose may be O-acetylated, so that up to two O-acetyl groups may be present on the oligomer [29].
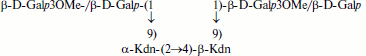 (2)
(2)Teichulosonic acid 3 was found in cell walls of 10 strains of the phylogenetic group S. setonii-griseus as well as in Streptomyces sp. VKM Ac-2090, S. macrosporus VKM Ac-777T, and Arthrobacter protophormiae VKM Ac-2104T [10-13, 28, 31]. The backbone β-Kdn residues were substituted at O8 with β-Glcp residues. Teichulosonic acid 4 isolated from cell walls of Arthrobacter spp. VKM Ac-2549 and VKM Ac-2550 [31] had a similar structure, differing from that of TULA 3 in the presence of N-acetyl-β-glucosamine residues that are also attached at O8 of β-Kdn:
where R = β-D-Glcp (TULA 3) or R = β-D-GlcpNAc (TULA 4).
The cell wall of Brevibacterium casei VKM Ac-2114T was found to contain TULA 5 [32] with disubstituted β-Kdn residues bearing β-Glcp residues at O8 and O9:
The polymeric chains of TULA-1–5 comprised >20 repeating units.
Teichulosonic acids containing Pse residues. Teichulosonic acids of this type were found in cell walls of representatives of the genus Kribbella [14, 20] and in Actinoplanes utahensis VKM Ac-674T (authors’ unpublished results). The basic chains of all polymers studied contain Pse residues acylated at N7 with 4-hydroxy- or 3,4-dihydroxybutanoyl groups, which are glycosylated at O4 with a Pse residue or another monosaccharide entering, along with Pse, into the main chain of the polymer. Usually the hydroxyl group at C4 of Pse bears a monosaccharide substituent, such as α-D-Galp, α-D-Galp3OMe, α-D-Galp2,3OMe, or β-L-Rhap.
Pse-containing teichulosonic acids were present, along with other glycopolymers such as teichuronic acids and neutral polysaccharides, in the cell walls of the studied actinomycetes (table). Their 13C NMR spectroscopic characteristics are given in Supplement Table 2.
Teichulosonic acid 6 was isolated from the cell wall of Kribbella sp. VKM Ac-2541 [14]. Its basic chain is built up of β-Pse residues with the 4-hydroxybutanoyl group as the N7 substituent, the hydroxyl group of the latter being glycosylated with a neighboring Pse residue. α-Galp3OMe or α-Galp2,3OMe were found as non-stoichiometric substituents at O4 of Pse. The following structure was established for the repeating unit of this polymer:
where R = H (45%), α-D-Galp3OMe (37%), or α-D-Galp2,3OMe (18%).
Teichulosonic acid 7 isolated from the cell walls of four strains of Kribbella spp. VKM (Ac-2500, Ac-2568, Ac-2572, Ac-2575) [20] was an irregular heteropolymer where the predominant fragments A with β-Pse and β-Galp residues in the basic chain and α-Galp3OMe or α-Galp2,3OMe residues in the side chain alternate irregularly with the fragments B:
where R = H, α-D-Galp3OMe or α-D-Galp2,3OMe.
Cell wall TULA-8 from Kribbella sp. VKM Ac-2527 [20] differed from TULA-7 in the presence of β-L-Rhap residues in addition to α-Galp3OMe and α-Galp2,3OMe residues.
A characteristic feature of TULA-9 identified in the cell wall of a sporangial actinomycete Actinoplanes utahensis VKM Ac-674T (authors’ unpublished results) was the presence of 3,4-dihydroxybutanoyl groups on β-Pse residues. Like TULA-7, it represented an irregular heteropolymer with alternating fragments A and B:
where R = α-D-Galp (80%) or Н (20%).
The polymeric chains of TULA-6–9 comprised 20 to 40 monosaccharide residues.
Summarizing the data of teichuronic acid and teichulosonic acids discovered not long ago, first of all it should be noted that it is the use of high-resolution NMR spectroscopy, as well as MALDI-TOF and ESI mass spectrometry, in combination with chemical degradation methods that enabled the progress in this field culminating in elucidation of about 20 novel polymer structures.
Investigations into cell wall glycopolymers of actinomycetes pioneered some 50 years ago by I. B. Naumova (1931-2003), who was the first to describe teichoic acids in this group of microorganisms [33]. Cell walls of more than 600 organisms belonging to different suborders, families, genera, and species of the order Actinomycetales have been studied to date. Novel teichoic acids, poly(glycosyl 1-phosphates), neutral polysaccharides as well as teichuronic and teichulosonic acids have been revealed. The latter two types of polymers are the topic of the present review.
Teichuronic acids considered to be typical polymers of cell walls of bacilli [1] are currently discovered in representatives of the order Actinomycetales [5, 9-14], which extends the former notion on the distribution of these polymers in the world of microorganisms.
Functioning of teichuronic acids, which are polyanionic polymers, is akin that of teichoic acids: they bind cations, impart negative charge to the cell surface, and control autolysin activity. However, it is believed [18, 34] that the biological roles of teichuronic and teichoic acids in the cell are different; for instance, the former are not involved in phage adsorption.
A novel class of glycopolymers, teichulosonic acids, derived their name from ulosonic acids, Kdn or Pse, contained in their repeating units [27]. By virtue of the negatively charged carboxyl groups of the ulosonic acids, the teichulosonic acids, like teichuronic acids, manifest pronounced anionic properties and supposedly play an analogous role in the vital activity of the microbial cell [1, 18, 35].
The oligomers/polymers of Kdn were first found in cell walls of phytopathogenic streptomycetes [10-13, 28-30]. It was suggested that the presence of Kdn-containing compounds on the cell surface plays a key role in pathogenesis favoring the phytopathogen cell adhesion to the host plant in the initial stages of infection. Polymers that contain Kdo (3-deoxy-D-manno-oct-2-ulosonic acid), also pertaining to the family of higher 3-deoxyald-2-ulosonic acids, have been reported to possess adhesive properties with respect to host plant cells [36].
Pse-containing cell wall teichulosonic acids were identified in representatives of the genera Kribbella and Actinoplanes [14, 20]. Various Pse derivatives were earlier described [35] as components of capsular and O-specific polysaccharides of more than 20 Gram-negative bacteria.
Presumably, one of the factors responsible for the pathogenicity of bacteria is associated with Kdn- and Pse-containing glycopolymers due to the structural similarity of these sugars with sialic acids, derivatives of 5-amino-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic (neuraminic) acid, which is an essential component of animal glycoproteins and glycolipids. In addition, the available data reveal similar stages in the biosynthesis of ald-2-ulosonic acids in microorganisms and sialic acids in higher organisms [35, 37, 38].
Recent studies [13, 39] have demonstrated the presence of teichulosonic acids in cell walls of representatives of soil microflora belonging to some genera of the order Actinomycetales (Streptomyces, Brevibacterium, Arthrobacter, Kribbella, Actinoplanes), which have not been reported to manifest phytopathogenicity. The presence of cell wall teichulosonic acids can testify to the ability of plant colonization by soil actinomycetes, which would result in either their symbiosis or development of pathogenesis.
At present, a polyphasic approach to the classification of microorganisms, in particular of representatives of the order Actinomycetales, is preferred. Studies on phenotypic characteristics, including structures of cell wall glycopolymers, are still an important issue, together with modern methods of genosystematics [40-42].
Analysis of teichoic acids of a vast massif of microorganisms of the order Actinomycetales have demonstrated that structural features of teichoic acids can be used as species-specific markers for representatives of some genera of actinomycetes, e.g. Streptomyces [43, 44], Nocardiopsis [45], Glycomyces [46, 47], and Nocardioides [48-50]. Recent studies have led to the conclusion that structural features of teichuronic and teichulosonic acids are also useful for taxonomy.
Studies of 25 strains of phytopathogenic streptomycetes revealed different cell wall glycopolymers, including Kdn-containing teichulosonic acids, in all the organisms (table). However, the nature and structures of the glycopolymers differ for representatives of different phylogenetic groups, S. violaceusniger, S. scabiei, and S. setonii-griseus, divided based on the nucleotide sequence 16S rRNA homology. Thus the group S. violaceusniger is characterized by the presence of cell wall glycerol teichoic acids and poly-Kdn with side-chain β-D-Galp residues; for the group S. scabiei, all representatives of which synthesize a phytotoxin thaxtomin [13], characteristic is the presence of glycerol teichoic acids and poly/oligo-Kdn with β-D-Galp and/or β-D-Galp3OMe residues, whereas ribitol teichoic acids, teichuronic acids, and β-D-Glcp-bearing poly-Kdn are typical of the group S. setonii-griseus.
The presence of strains (groups of strains) with varying nature and structures of the glycopolymers (subgroups, see table, column 1) in the cell walls of each of the aforementioned phylogenetic groups may suggest that they pertain to different species/subspecies. Similar conclusions can be inferred from the analysis of cell wall polymers of representatives of the genus Kribbella not assigned to the species (table). Nine strains of this genus studied were heterogeneous as regards the nature and structures of cell wall glycopolymers, which can also suggest the existence of several species/subspecies.
Thus, the data surveyed are not only evidence of structural diversity and abundance of cell wall teichuronic and teichulosonic acids of actinomycetes, but they are also useful for the solution of taxonomic problems associated with differentiation of closely related representatives of the phylogenetic clusters of streptomycetes and Kribbella strains at the phenotypic level.
REFERENCES
1.Naumova, I. B., and Shashkov, A. S. (1997)
Biochemistry (Moscow), 62, 809-840.
2.Diaz-Maurino, T., and Perkins, H. R. (1974) J.
Gen. Microbiol., 80, 533-539.
3.Petersen, B. O., Sara, M., Madler, C., Mayer, H.
F., Sleytr, U. B., Pabst, M., Puchberger, M., Krause, E., Hofinger, A.,
Duus, J. O., and Kosma, P. (2008) Carbohydr. Res., 343,
1346-1358.
4.Iwasaki, H., Araki, Y., Kaya, S., and Ito, E.
(1989) Eur. J. Biochem., 178, 643-648.
5.Tul’skaya, E. M., Shashkov, A. S.,
Senchenkova, S. N., Akimov, V. N., Bueva, O. V., Stupar’, O. S.,
and Evtushenko, L. I. (2007) Russ. J. Bioorg. Chem.,
33, 251-257.
6.Yoneyama, T., Araki, Y., and Ito, E. (1984) Eur.
J. Biochem., 141, 83-89.
7.Ward, J. B. (1981) Microbiol. Rev.,
45, 211-243.
8.Janczura, E., Perkins, H. E., and Rogers, H. J.
(1961) Biochem. J., 80, 82-93.
9.Shashkov, A. S., Kochanowski, H., Kozlova, Yu. I.,
Streshinskaya, G. M., Terekhova, L. P., and Galatenko, O. A. (1994)
Biochim. Biophys. Acta, 1201, 333-338.
10.Kosmachevskaya, L. N., Streshinskaya, G. M.,
Evtushenko, L. I., Bueva, O. V., Denisenko, V. A., Naumova, I. B., and
Stackebrandt, E. (2002) Eur. J. Biochem., 269,
6020-6025.
11.Shashkov, A. S., Kozlova, Yu. I., Streshinskaya,
G. M., Kosmachevskaya, L. N., Bueva, O. V., Evtushenko, L. I., and
Naumova, I. B. (2001) Microbiology, 70, 413-421.
12.Tul’skaya, E. M., Shashkov, A. S.,
Evtushenko, L. I., Stomakhin, A. A., Denisenko, V. A., Ivanyuk, V. G.,
and Naumova, I. B. (2003) in Proc. Int. Commemorative Research and
Practice Conf. Dedicated to 75th Anniversary of the Institute of Potato
Growing of the National Academy of Sciences of Belarus [in
Russian], Pt. II, Belarusskaya Nauka, Minsk, pp. 200-210.
13.Tul’skaya, E. M., Streshinskaya, G. M.,
Shashkov, A. S., Bueva, O. V., Baryshnikova, L. M., and Evtushenko, L.
I. (2010) in State of the Art and Prospects for the Development of
Microbiology and Biotechnology, Proc. VII Int. Conf. [in Russian],
Minsk, May 31-June 4, 2010, Belarusskaya Nauka, Minsk, pp. 469-471.
14.Shashkov, A. S., Tul’skaya, E. M.,
Streshinskaya, G. M., Senchenkova, S. N., Avtukh, A. N., and
Evtushenko, L. I. (2009) Carbohydr. Res., 344,
2255-2262.
15.Ellwood, D. C., and Tempest, D. W. (1969)
Biochem. J., 111, 1-5.
16.White, P. (1977) J. Gen. Microbiol.,
102, 435-439.
17.Lifley, M. R., Tarelli, E., and Baddiley, J.
(1980) Biochem. J., 191, 305-318.
18.Hughes, R. S., Pavlik, J. G., Rogers, H. J., and
Tanner, P. J. (1968) Nature (London), 219, 642-644.
19.Seltmann, G., and Holst, O. (2002) The
Bacterial Cell Wall, Springer-Verlag, Berlin, pp. 133-144.
20.Tul’skaya, E. M., Streshinskaya, G. M.,
Shashkov, A. S., Senchenkova, S. N., Avtukh, A. N., Baryshnikova, L.
M., and Evtushenko, L. I. (2011) Carbohydr. Res., 346,
doi: 10.1016/j.carres.2011.06.003.
21.Wright, J., and Heckels, J. E. (1975) Biochem.
J., 147, 187-189.
22.Hase, S., and Matsushima, Y. (1972) J.
Biochem. (Tokyo), 72, 1117-1128.
23.Din, N. U., and Jeanloz, R. W. (1976)
Carbohydr. Res., 47, 245-260.
24.Johnson, S. D., Lacher, K. P., and Anderson, J.
S. (1981) Biochemistry, 20, 4781-4785.
25.Wu, T. C., and Park, J. T. (1971) J.
Bacteriol., 108, 874-884.
26.Nagaoka, M., Kamisango K., Fujii, H., Uchikawa,
K., Sekikawa, I., and Azuma, I. (1985) J. Biochem.
(Tokyo), 97, 1669-1678.
27.Knirel, Y. A. (2009) in Progress in the
Synthesis of Complex Carbohydrate Chains of Plant and Microbial
Polysaccharides (Nifantiev, N. E., ed.) Transworld Research
Network, Kerala, India, pp. 181-198.
28.Shashkov, A. S., Streshinskaya, G. M.,
Kosmachevskaya, L. N., Evtushenko, L. I., and Naumova, I. B. (2000)
Mendeleev Commun., 5, 167-168.
29.Shashkov, A. S., Tul’skaya, E. M.,
Evtushenko, L. I., Denisenko, V. A., Ivanyuk, V. G., Stomakhin, A. A.,
Naumova, I. B., and Stackebrandt, E. (2002) Carbohydr. Res.,
337, 2255-2261.
30.Tul’skaya, E. M., Shashkov, A. S., Bueva,
O. V., and Evtushenko, L. I. (2007) Microbiology, 72,
39-44.
31.Potekhina, N. V., Shashkov, A. S., Senchenkova,
S. N., Kozlova, Yu. I., Streshinskaya, G. M., Dorofeeva, L. V.,
Yastrebova, O. V., and Evtushenko, L. I. (2010) State of the Art and
Prospects for the Development of Microbiology and Biotechnology,
Proc. VII Int. Conf. [in Russian], Minsk, May 31-June 4, 2010,
Belarusskaya Nauka, Minsk, pp. 148-150.
32.Potekhina, N. V., Evtushenko, L. I., Senchenkova,
S. N., and Shashkov, A. S. (2007) Russ. J. Bioorg. Chem.,
33, 66-72.
33.Naumova, I. B., Belozersky, A. N., and Shafikova,
F. A. (1962) Dokl. Akad. Nauk SSSR, 143,
730-734.
34.Bhavsar, A. P., Erdman, L. K., Schertzer, J. W.,
and Brown, E. D. (2004) J. Bacteriol., 186,
7865-7873.
35.Knirel, Yu. A., Shashkov, A. S., Tsvetkov, Yu.
E., Jansson, P-.E., and Zahringer, U. (2003) Adv. Carbohydr. Chem.
Biochem., 58, 371-417.
36.Reuhs, B. L., Kim, J. S., and Matthysse, A. G.
(1997) J. Bacteriol., 179, 5372-5379.
37.Inoue, S., and Kitajima, K. (2006) Glycoconj.
J., 23, 277-290.
38.Lewis, A. L., Desa, N., Hansen, E. E., Knirel,
Yu. A., Gordon, J. I., Gagneux, P., Nizet, V., and Varki, A. (2009)
Proc. Natl. Acad. Sci. USA, 106, 13552-13557.
39.Tul’skaya, E. M., Shashkov, A. S.,
Streshinskaya, G. M., Potekhina, N. V., Kozlova, Yu. I., Senchenkova,
S. N., and Evtushenko, L. I. (2009) in State-of-the Art Problems of
Ecology and Biotechnology of Microorganisms, Proc. All-Russian
Symp. [in Russian], MAKS Press, Moscow, p. 182.
40.Stackebrandt, E. (2006) in The
Prokaryotes: A Handbook on the Biology of Bacteria, Vol.
1 (Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., and
Dworkin, M., eds.) 3rd Edn., Springer, Berlin, pp. 29-57.
41.Kampfer, P. (2010) Int. J. Syst. Evol.
Microbiol., 60, 7.
42.Tindall, B. J., Rossello-Mora, R., Busse, H.-J.,
Ludwig, W., and Kampfer, P. (2010) Int. J. Syst. Evol.
Microbiol., 60, 249-266.
43.Streshinskaya, G. M., Shashkov, A. S., and
Naumova, I. B. (1995) Biochemistry (Moscow), 60,
963-970.
44.Streshinskaya, G. M., Kozlova, Yu. I., Shashkov,
A. S., Evtushenko, L. I., and Naumova, I. B. (2003)
Microbiology, 72, 455-460.
45.Naumova, I. B., Shashkov, A. S., Tul’skaya,
E. M., Streshinskaya, G. M., Kozlova, Y. I., Potekhina, N. V.,
Evtushenko, L. I., and Stackebrandt, E. (2001) FEMS Microbiol.
Rev., 25, 269-284.
46.Potekhina, N. V., Tul’skaya, E. M.,
Naumova, I. B., Shashkov, A. S., and Evtushenko, L. I. (1993) Eur.
J. Biochem., 218, 371-375.
47.Tul’skaya, E. M., Potekhina, N. V.,
Naumova, I. B., Shashkov, A. S., and Evtushenko, L. I. (1993)
Microbiology, 62, 557-559.
48.Shashkov, A. S., Tul’skaya, E. M.,
Evtushenko, L. I., and Naumova, I. B. (1999) Biochemistry
(Moscow), 64, 1305-1309.
49.Shashkov, A. S., Tul’skaya, E. M.,
Evtushenko, L. I., Grachev, A. A., and Naumova, I. B. (2000)
Biochemistry (Moscow), 65, 509-514.
50.Tul’skaya, E. M., Krausova, V. I., Gavrish,
E. Yu., Shashkov, A. S., Evtushenko, L. I., and Naumova, I. B. (2003)
in Abst. Book of the 1st FEMS Congr. European Microbiologists,
Ljubljana, Slovenia, June 29-July 3, p. 357.
51.Tul’skaya, E. M., Vylegzhanina, E. S.,
Streshinskaya, G. M., Shashkov, A. S., and Naumova, I. B. (1991)
Biochim. Biophys. Acta, 1074, 237-242.
Supplementary Tables (MS Word)