REVIEW: On the Programmed/Non-Programmed Aging Controversy
T. C. Goldsmith
Azinet LLC, Box 239 Crownsville, MD 21032 USA; fax: 1-443-403-2283; E-mail: tgoldsmith@azinet.com
Received January 10, 2012
The programmed vs. non-programmed aging controversy has now existed in some form for at least 150 years. For much of the XX century, it was almost universally believed that evolution theory prohibited programmed (adaptive) aging in mammals and there was little direct experimental or observational evidence favoring it. More recently, multiple new evolutionary mechanics concepts that support programmed aging and steadily increasing direct evidence favoring it overwhelmingly support the existence of programmed aging in humans and other organisms. This issue is important because the different theories suggest very different mechanisms for the aging process that in turn suggest very different paths toward treating and preventing age-related diseases.
KEY WORDS: aging, senescence, evolution, gerontologyDOI: 10.1134/S000629791207005X
Because aging and lifespan characteristics vary enormously between even very similar species, it has long been accepted that intrinsic organism lifespan is genetically determined and developed through an evolutionary process in a way similar to the one that determines other species-specific characteristics. If lifespans were generically imposed by some fundamental limitation such as a law of physics or chemistry, we would not see the observed extreme variation in lifespan between similar species that possess similar biochemistry and similar exposure to generic deteriorative processes.
The programmed (adaptive) aging concept holds that organisms possess potentially complex evolved mechanisms that exist for the purpose of pro-actively limiting the organism’s lifespan beyond a species-specific age. Non-programmed evolutionary theories of aging contend that aging passively and incidentally results from lack of evolutionary force toward continuing life beyond a species-specific age.
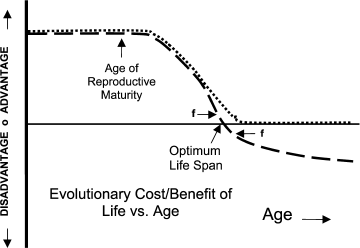
As shown in the figure, evolutionary non-programmed theories of aging depend on the idea that the net (of any tradeoffs) evolutionary force toward living and reproducing beyond some species-specific age is effectively zero (dotted curve). If this force was even slightly positive (living longer creates evolutionary benefit), presumably the organism would have evolved a longer lifespan. If the force beyond that age was even slightly negative (continuing life creates evolutionary disadvantage – dashed curve), presumably the organism would have evolved pro-active means for limiting life, i.e. programmed lifespan limiting mechanisms. Note that in the latter case there is evolutionary force (f) both toward maintaining life prior to and limiting life after the optimum lifespan. The evolutionary difference between non-programmed and programmed aging is therefore essentially the difference between “effectively zero” and “at least slightly negative”.
In both concepts, species-unique intrinsic and extrinsic factors clearly influence evolved lifespan. The most important intrinsic factor is the age at which the organism is initially capable of reproducing. Everybody agrees that lifespan must match or exceed this age. The age at which the organism is developmentally mature and fully expresses adult characteristics is another factor. The evolution of adult characteristics requires organisms to live long enough to become adults and express adult characteristics. Animals that nurture or protect their young would need additional lifespan to perform that function. Extrinsic factors that plausibly affect lifespan include degree of predation, existence of famine or drought conditions, population density, and environmental factors. Note that the extrinsic factors can change depending on temporary or local conditions and that an organism design capable of accommodating its lifespan to these temporary or local changes would have an evolutionary advantage. Note also that in mammals and other more complex organisms, age of reproductive maturity (and consequent reproductive behavior) is itself controlled by a complex mechanism capable of detecting and accommodating to external conditions such as seasons. An organism would benefit from the ability to accommodate its lifespan to such changes in its age of reproductive maturity. Regulated programmed aging refers to an organism design capable of adjusting lifespan to accommodate temporary or local extrinsic or intrinsic conditions.
Evolutionary cost or benefit of continued life as a function of age. Dotted line, non-programmed aging theory – net benefit of continued life declines to zero. Dashed line, programmed aging theory – life beyond optimum lifespan produces evolutionary disadvantage
Because they relate evolved lifespan to multiple species-unique factors, evolutionary programmed and non-programmed theories provide a much better match to multi-species lifespan observations than the generic damage or “wear and tear” theories.
INDIVIDUAL BENEFIT ISSUE
For much of the XX century, it was very widely thought that only individual benefit or disadvantage could influence the evolution process. According to this concept, any evolved organism design characteristic must provide a net benefit to the ability of the possessing individual organisms (or their direct descendants) to survive or reproduce. It was further widely thought that only in special cases, specifically excluding gradually aging mammals, would there exist an individual benefit to a purposely limited lifespan. Salmon are often cited as an example of such a special case. Salt-water salmon spawn in the restricted environment of a fresh-water stream. If the adult salmon were programmed to die soon after spawning (as observed), their corpses could plausibly provide food for their direct descendants, creating an individual benefit from death, per se, and driving the evolution of a suicide mechanism. Salmon that possessed the suicide mechanism could therefore have an individual benefit advantage over those that survived spawning and died later after parents and direct descendants were widely dispersed. No such individual advantage of death or aging is apparent for most animals.
Beginning in 1952, a series of non-programmed mammal aging theories appeared based on the idea proposed by Medawar [] that the net individual benefit of continuing life declines to zero at a species-specific age related to reproductive maturity. These included the mutation accumulation theory [1] (Medawar), antagonistic pleiotropy theory [] (Williams), and disposable soma theory [] (Kirkwood). This effort has not been notably successful despite its long duration. The theories attack each other, and they have many apparent logical flaws []. None has achieved general consensus.
However, beginning in 1962, a series of evolutionary diffuse benefit theories appeared. All of these theories contend that a diffuse (non-individual) benefit can offset individual disadvantage and cause evolution of an organism design characteristic that produces a wider benefit even if it also produces some degree of individual disadvantage. The diffuse benefit theories now include:
– group selection [] (benefit to survival of a group can offset individual disadvantage);
– kin selection [] (benefit to close relatives can offset individual disadvantage);
– gene-oriented selection [] (benefit to propagation of genes common to a population can offset individual disadvantage);
– evolvability [] (benefit to the evolution process can offset individual disadvantage).
Note that these theories were developed in efforts to explain observed discrepancies between observations and traditional individual-benefit-only theory other than aging and lifespan. Altruism or observed inherited animal behaviors that operate against the individual interest of an animal but simultaneously provide plausible group benefit was a major early incentive for developing diffuse theories. Other apparent discrepancies between observations and the individual benefit concept include observation of apparently unnecessarily late reproductive maturity, some mating behaviors, and the individually adverse nature of sexual reproduction.
Since about 1950 there has been an explosive and continuing increase in knowledge regarding biological inheritance mechanisms, which are crucial to evolutionary mechanics because inheritable changes in organism designs are propagated and retained through biological inheritance. The diffuse theories are all either directly based on or greatly supported by these discoveries.
Specific mammal programmed aging theories have been developed based on group selection [], kin selection [], and evolvability [, ]. Unlike the individual benefit aging theories, these theories contend that design-limited organism lifespan is generally beneficial and that species that do not need programmed lifespan management (if any) are the special cases. This is an important difference in emphasis: Non-programmed proponents contend programmed aging only applies in special cases and tend to discount non-mammal observations as irrelevant to mammal aging. Programmed aging proponents contend that species have a general need for lifespan control and consequently data from a wide variety of species is relevant. Some programmed aging theories [11, 12] contend that mammals and other complex organisms have a greater need for programmed lifespan management than simpler organisms. There has been little scientific objection to the many specific proposed diffuse benefits of a design-limited lifespan. Objections have centered on propagation issues described below.
ARGUMENTS FOR AND AGAINST DIFFUSE BENEFIT THEORIES
A classical argument against the diffuse theories is that they all appear to require a tradeoff between a long-term diffuse benefit and a short-term individual disadvantage (e.g. between reduced probability of species extinction and reduced probability of individual survival and reproduction). This raises an obvious evolutionary mechanics question: How would an individually adverse organism design characteristic propagate and be retained long enough for the long-term benefit to be achieved? Experience with selective breeding shows that very large phenotypic changes can be produced in a very short time. Would not individual advantage thus be selected over any amount of long-term benefit? Perhaps diffuse theories only work for relatively short-term benefit such as benefit to small groups, small isolated populations, etc.
In 1957 Williams [2] suggested a solution to this problem in aid of his non-programmed aging theory that apparently works even better for programmed aging and for diffuse benefit theories generally [4]: In selective breeding, the breeder is usually interested in enhancing or attenuating some specific organism characteristic and relatively unconcerned about inadvertent associated changes to other design parameters. In contrast, natural selection is “concerned” with the combined net effect of all of an organism’s inherited design characteristics. Williams’ problem was that he believed that indefinitely continued life (and reproduction), per se, was generally at least mildly individually beneficial. How then to explain why organisms would arrive at an age at which further life and reproduction would have zero net individual benefit? Williams suggested that an individually adverse design characteristic could be rigidly linked to an individually beneficial design characteristic (or characteristics) in such a way that the evolution process could not obtain the beneficial effect(s) without incurring the adverse effect, in this case, aging. The linked benefit could be to any organism characteristic that aided younger organisms in surviving or reproducing, because, per the figure, the evolutionary value of survival or reproduction in younger organisms is greater. The beneficial effect(s), if sufficient, would then protect the adverse characteristic from being removed by natural selection and result in the required zero net individual benefit at the target age. Williams cited antagonistic pleiotropy (based on genomics discoveries) as the linking mechanism. Because there would have always, since primordial time, been evolutionary force toward breaking the linkage and allowing the beneficial characteristic without the adverse effect of limited lifespan, Williams had to assume that the linkage was perfectly rigid, that is unbreakable, despite operation of evolutionary mechanisms for any duration.
Analysis [4] of subsequent genetics discoveries shows that not only is antagonistic pleiotropy a valid source of linkage, there are many other sources of linkage associated with various aspects of genomic design. Further, different sources have different degrees of rigidity defined as a measure of the difficulty and therefore the time required for the evolution process to remove the linkage. This analysis suggests that antagonistic pleiotropy, per se, is not sufficiently rigid to protect an adverse characteristic from being selected out during a very long evolutionary time period but would be sufficient to protect an individually adverse characteristic having a diffuse benefit from being selected out during the time required for the diffuse benefit to be effective, even a species-level benefit. Surviving species could then pass the linked characteristics to their descendants.
Another counter-argument is associated with evolvability. The evolvability proposal is that organism design characteristics that enhance the evolution process (i.e. the rate at which an organism could adapt to a change in its external world) can be evolved and retained despite some degree of individual disadvantage. Evolvability is sometimes seen as benefiting the species or future descendant species and therefore producing a very long-term benefit. However, analysis [4] shows that an evolvability characteristic affects the preconditions required for the evolution process to operate and therefore is effective regardless of the evolutionary timeframe contemplated. A benefit to the evolution process operates on the same time-scale as natural selection.
EMPIRICAL EVIDENCE AND AGING THEORIES
Proponents of non-programmed theories typically contend that any examination and interpretation of empirical evidence concerning the programmed/non-programmed issue should be very heavily biased in favor of non-programmed aging because of evolutionary considerations. In 2004, Hayflick et al. [13] said that their evolutionary mechanics concept made human programmed aging “impossible” and implied that any empirical evidence favoring programmed aging such as genes that cause aging should be derogated, discounted, and disregarded. Following this philosophy, the development of a biological aging theory is limited to devising the least implausible non-programmed theory and then constructing the least implausible interpretations of empirical evidence that support the theory. Such a philosophy does not support funding or performing experiments designed to find evidence of programmed aging or designed to distinguish between programmed and non-programmed theories and is therefore substantially a self-fulfilling prophecy. In 2011, Kirkwood and Melov similarly suggested [14] that because of their evolutionary concepts, empirical evidence of programmed aging would have to overcome “high barriers” to acceptance not required of non-programmed theories. They went on to say that, in their opinion, a belief in programmed mammal aging was equivalent to a belief that “the sun orbits the Earth”. One can easily imagine the chilling effect such ideological pronouncements by senior scientists might have on research and funding.
The reality is that in the last 50 years our collective certainty with respect to most aspects of evolution theory has indeed steadily increased. However, during the same period, our certainty regarding details that are absolutely crucial to evolutionary aging theory has obviously decreased. It is increasingly clear that the rich complexity in genomic designs exposed by genetics research affects evolutionary mechanics issues that directly bear on the programmed/non-programmed question. Additionally, as described above, distinguishing between evolutionary aging theories requires hair-splitting the difference between “effectively zero” and “at least slightly negative”. With regard to aging theory, “evolution theory” no longer provides a scientifically acceptable rationale for rejecting empirical evidence or biasing its interpretation. There now exists a long list of observations and experiments that have been cited as supporting programmed aging in mammals [4] including genes that cause aging, negligible senescence, progerias, caloric restriction effects, stress effects, regulated aging in worms, and octopus suicide. Those interested in this issue should compare the regulated programmed aging explanation (e.g. [4]) with the non-programmed aging explanation, if one exists (e.g. [14]) in regard to each of these observations. In general, the reader will find that the non-programmed explanation is much more convoluted and implausible, and in some cases depends on assumptions for which no evidence is presented.
Understanding aging mechanisms is obviously critical to our ability to prevent and treat age-related diseases. Programmed aging theories predict the existence of opportunities not predicted by non-programmed theories. For example, if aging is purposely imposed by a biological mechanism, interfering with the operation of that mechanism is a likely possibility. Such a mechanism plausibly includes a clock mechanism and provisions for signaling, which offer points at which intervention might be attempted. If aging is substantially the result of a regulated mechanism, then detection, signaling, and other mechanics involved in regulation represent additional points at which intervention could be attempted.
The cause of aging is a serious issue having manifest impact on public health and deserves careful attention by a wide scientific community.
REFERENCES
1.Medawar, P. (1952) An Unsolved Problem of
Biology, H.K. Lewis & Co., London.
2.Williams, G. (1957) Evolution, 11,
398-411.
3.Kirkwood, T., and Holliday, R. (1979) Proc. Roy.
Soc. London B, 205, 531-546.
4.Goldsmith, T. (2011) Aging by Design, Azinet
Press, Annapolis, ISBN 0-9788709-3-X.
5.Wynne-Edwards, V. (1962) Animal Dispersion in
Relation to Social Behaviour, Oliver & Boyd, Edinburgh.
6.Hamilton, W. (1964) J. Theor. Biol.,
7, 1-16.
7.Dawkins, R. (1976) The Selfish Gene, Oxford
University Press, ISBN 0-19-286092-5.
8.Wagner, G. (1996) Evolution, 50,
967-976.
9.Mitteldorf, J. (2004) Evol. Ecol. Res.,
6, 1-17.
0.Libertini, G. (1988) J. Theor. Biol.,
132, 145-162.
1.Goldsmith, T. (2008) J. Theor. Biol.,
252, 764-768.
2.Skulachev, V. (1997) Biochemistry
(Moscow), 62, 1191-1195.
3.Olshansky, S., Hayflick, L., and Carnes, B. (2004)
Scientific American.
4.Kirkwood, T., and Melov, S. (2011) Curr.
Biol., 21, R701-R707.