Superoxide Scavenging Activity of Plastoquinone Derivative 10-(6′-Plastoquinonyl)decyltriphenylphosphonium (SkQ1)
V. A. Chistyakov1,2*, E. V. Prazdnova2, L. V. Gutnikova2, M. A. Sazykina2, and I. S. Sazykin2
1Rostov State Medical University, Pereulok Nakhichevanskii 29, 344022 Rostov-on-Don, Russia; E-mail: vladimirchi@yandex.ru2Research Institute of Biology, Southern Federal University, pr. Stachki 194/1, 344090 Rostov-on-Don, Russia; fax: (863) 299-5661
* To whom correspondence should be addressed.
Received April 1, 2012
The plastoquinone derivative 10-(6′-plastoquinonyl)decyltriphenylphosphonium (SkQ1) has the ability to scavenge superoxide anion radical. This ability is manifested both in vitro and in vivo in experiments with the bacterium Escherichia coli. The protective effect of SkQ1 in vivo significantly exceeds that of ascorbic acid.
KEY WORDS: Skulachev ions, SkQ1, superoxide anion radical, superoxide scavenging activity, E. coliDOI: 10.1134/S0006297912070103
Abbreviations: HBO, hyperbaric oxygenation; O2–., superoxide anion radical; SkQ1, plastoquinone derivative 10-(6′-plastoquinonyl)decyltriphenylphosphonium; SOD, superoxide dismutase.
The lipophilic cation
10-(6′-plastoquinonyl)decyltriphenylphosphonium (SkQ1) is the
most effective geroprotector known today [1]. The
ability of SkQ1 to reduce the intensity of pathological processes
connected with ROS generation forms the basis of this activity. The
activity has been found in tens of models of different degrees of
complexity, both in vitro and in vivo. Nevertheless, the
biochemical mechanisms forming the basis of the antioxidant activity of
SkQ1 are not completely studied. In particular, data on the ability of
SkQ1 to “intercept” superoxide anion radical
(O2–·) are quite fragmentary. It is
known that O2–. is the primary free-radical
product generated during respiratory chain functioning. Its production
turns the mitochondria into the “dirty places in a cell”
and starts the processes causing phenoptosis [2].
The purpose of this work was to study the ability of SkQ1 to
“intercept” O2–..
Superoxide scavenging activity of SkQ1 was determined in vitro by means of a standard test system (SOD determination kit 19160; Sigma, USA). Optical density values were measured using the microplate reader of an Alisei automatic analyzer (Radim, Italy). Superoxide dismutase (SOD) at concentrations 1-200 units/ml (Sigma) was used for calibration. Superoxide scavenging activity of SkQ1 was calculated by the method of Fridovich [3].
In vivo superoxide-scavenging activity was evaluated according to the decrease in E. coli Sox-operon induction caused by exposure of the bacteria to pure oxygen with 0.3 MPa for 1 h. Escherichia coli MG1655 (pSoxS-lux) strain, provided by I. V. Manukhov (State Scientific Center Genetika, Moscow) was the object of the study. Cells of this strain are a lux-biosensor of Sox-operon induction by luminescence intensification [4]. For luminescence measurements, an LM-01A automatic microplate luminometer (Immunotech, Czech Republic) was used. Measurements were started 10 min after taking samples out of the pressure chamber, and then were carried out every 10 min for 120 min. For evaluation of the influence of the studied factors on Sox-operon expression, the induction factor (Is) was calculated according to the formula:
Is = (Le/Lk) – 1, (1)
where Lk and Le are luminescence intensities of control and experimental samples, respectively.
A statistically significant excess of Le over Lk estimated with the t-criterion was considered as a sign of significant influence on the induction effect.
Protective activity (P, %) was calculated taking induction into account in the presence of corresponding protector concentrations:
P = (1 – (Ia/Ip))·100%, (2)
where Ia and Ip are induction factors of the SOS-response with the investigated influence in the presence of protector and in the control sample, respectively.
To characterize the protective activity of the studied concentration of the substance, the mean value of P during the whole duration of measurements was used.
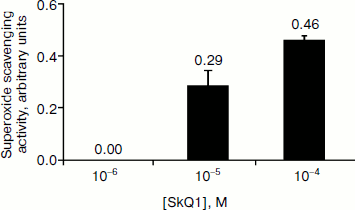
As seen in Fig. 1, SkQ1 in vitro at 10–5 and 10–4 M concentrations shows superoxide scavenging activity (0.29 and 0.46 arbitrary unit). It should be pointed out that the estimation method forming the basis of the test system registers “interception” of superoxide anion radical generated in the xanthine oxidase–xanthine system. This generation system is one of the most reliable according to reproducibility of results and, therefore, it is widely used. However it is meant for detection of high activity, characteristic for SOD enzyme. The sensitivity of the test was found to be insufficient for detection of superoxide scavenging activity of ascorbic acid (data not shown), which is reliably registered with more sensitive resonance methods [5]. Thus, the superoxide scavenging activity of SkQ1 according to the test in vitro can be characterized as high.
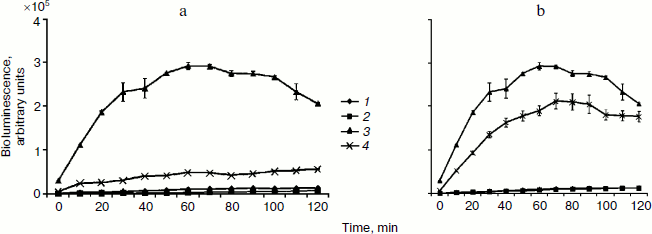
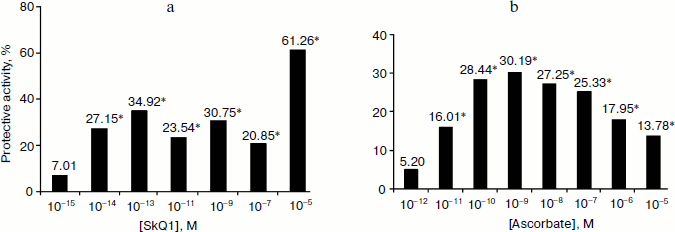
Processing of bacteria with oxygen under pressure causes a strong induction of the Sox-operon (Fig. 2), which is considered to be the consequence of growth of the intercellular level of superoxide anion radical [4]. Introduction of SkQ1 at 10–5 M concentration considerably reduces Sox-induction (on average by 61.3%) (Fig. 2a). Statistically significant effects are registered even for concentrations as low as 10–14 M (Fig. 2b). The comparative data on the ability of different concentrations of ascorbic acid and SkQ1 to suppress O2–. generation caused by hyperbaric oxygenation (HBO) are shown in Fig. 3. This figure shows higher superoxide scavenging activity is characteristic for SkQ1 in vivo. Minimum concentrations of SkQ1 and ascorbate that show the effect are 10–14 and 10–11 M, respectively. Maximum protective effect reached with SkQ1 and ascorbate is 61.3 and 30.2%, respectively. Superoxide scavenging activity of SkQ1 is manifested in vitro at concentrations much higher than those in vivo. This is apparently connected, on one hand, with accumulation of the substance in bacterial cells by means of the electrochemical mechanism described in detail, and, on the other hand, with formation of oxidation-reduction cycles conjugated with the respiratory chain enzymes. As a result, the balance of the oxidized and reduced forms remains constant without rigid dependence on concentration [1].Fig. 1. Superoxide scavenging activity of SkQ1 in vitro at 10–6-10–4 M concentrations.
Fig. 2. Escherichia coli MG1655 (pSoxS-lux) strain bioluminescence after exposure to hyperbaric oxygenation (HBO) of 0.3 MPa for 1 h in the presence of SkQ1 at 10–5 M (a) and 10–14 M (b) concentrations: 1) control; 2) SkQ1; 3) HBO; 4) HBO + SkQ1.
Thus, SkQ1 is able to intercept superoxide anion radical. This ability is demonstrated both in vitro and in vivo in experiments with aerobic bacteria. Similar processes apparently occur in mitochondria. This is indirectly proved by numerous data on hydrogen peroxide (which is the main product of superoxide radical dismutation) generation decrease in mitochondria in the presence of SkQ1 [1]. The HBO bacterial model is promising for express screening of new Skulachev ions with antioxidant load.Fig. 3. SkQ1 (a) and ascorbate (b) protective activity at exposure to hyperbaric oxygenation of 0.3 MPa during 1 h. * Statistically significant effects (p < 0.05).
The authors express their sincere gratitude to V. P. Skulachev for help in organizing the research and interpretation of experimental results.
This work was completed with the financial support of the Institute of Mitoengineering of Moscow State University, the Ministry of Education and Science of the Russian Federation, and the Ministry of Health and Social Development of the Russian Federation.
REFERENCES
1.Skulachev, M. V., Antonenko, Y. N., Anisimov, V.
N., Chernyak, B. V., Cherepanov, D. A., Chistyakov, V. A., Egorov, M.
V., Kolosova, N. G., Korshunova, G. A., Lyamzaev, K. G., Plotnikov, E.
Y., Roginsky, V. A., Savchenko, A. Y., Severina, I. I., Severin, F. F.,
Shkurat, T. P., Tashlitsky, V. N., Shidlovsky, K. M., Vyssokikh, M. Y.,
Zamyatnin, A. A., Jr., Zorov, D. B., and Skulachev, V. P. (2011)
Curr. Drug Targets, 12, 800-826.
2.Skulachev, V. P. (2005) IUBMB Life,
57, 305-310.
3.Fridovich, I. (1979) Free Radicals in
Biology [Russian translation], Mir, Moscow, pp. 190-226.
4.Zavilgelsky, G. B., Kotova, V. Yu., and Manukhov,
I. V. (2007) Mutat. Res., 634, 172-176.
5.Wagner, A. E., Huebbe, P., Konishi, T., Rahman, M.
M., Nakahara, N., Matsugo, S., and Rimbach, G. (2008) J. Agric. Food
Chem., 56, 11694-11699.